Rust and scale are the silent enemies of carbon steel pipes, compromising their efficiency and longevity. For those in the industrial sector, understanding the best methods to combat these issues is crucial. This comprehensive guide dives deep into the world of chemical cleaning, offering an intricate look at various techniques designed to maintain and enhance the performance of carbon steel pipes. From the pickling process to the use of hydrochloric acid, this article meticulously outlines step-by-step procedures, benefits, and essential safety protocols. Whether you’re seeking to remove stubborn rust or scale, or simply aiming to understand the intricacies of chemical cleaning, this guide promises to be an invaluable resource. Ready to uncover the secrets to pristine pipes? Let’s get started.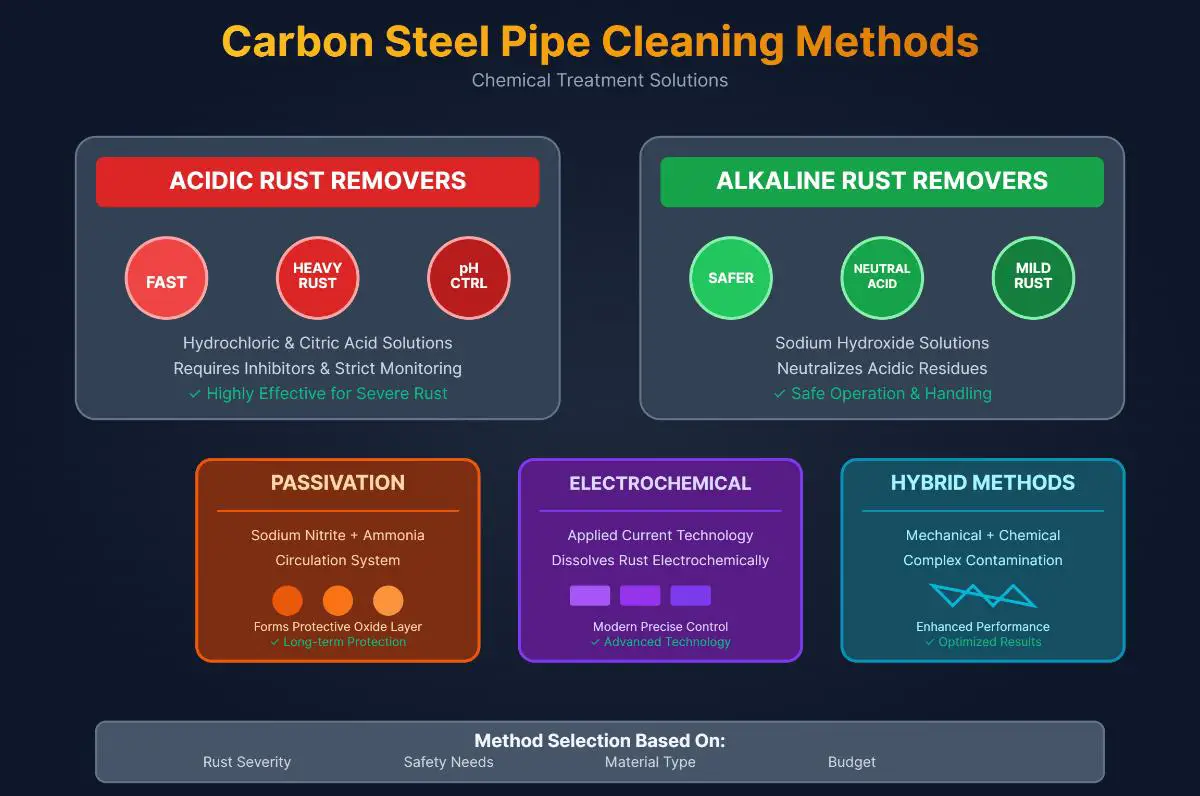
Chemical cleaning is essential for keeping carbon steel pipes efficient and long-lasting. This process removes various contaminants, such as rust, scale, and organic residues, which can compromise the structural integrity and performance of the pipes. By ensuring the pipes are clean, chemical cleaning helps prevent corrosion, blockages, and other issues that can lead to costly repairs and downtime.
Degreasing is the first step in the chemical cleaning process. It uses solutions to remove oils, greases, and other residues from the pipes. This step is essential to prepare the pipes for subsequent cleaning stages. Typically, hot water rinsing follows degreasing to ensure all contaminants are thoroughly removed.
Pickling is a crucial stage where acid solutions, such as hydrochloric acid, sulfuric acid, or phosphoric acid, are used to dissolve rust, scale, and oxide layers on the pipe surface. Inhibitors are added during pickling to protect the pipe’s base metal from the acid. This step is vital for restoring the pipe’s surface and enhancing its cleanliness and durability.
After the pickling process, neutralization is performed to remove any residual acidic substances from the pipes. Neutralizing solutions, often containing sodium phosphate or similar agents, are used to adjust the pH levels and ensure the pipes are free from acidic residues. This step is important to prevent corrosion and prepare the pipes for further treatments.
Passivation involves treating the pipe surface with a solution containing sodium phosphate and sodium nitrite. This treatment creates a protective layer that extends the pipes’ lifespan and boosts corrosion resistance. Passivation is a critical step for ensuring the pipes maintain their structural integrity over time.
The final step in the chemical cleaning process is drying. This step eliminates moisture from the pipes, which is crucial to prevent corrosion. Blower fans are often used to achieve thorough drying, ensuring that the pipes are completely free from water before they are put back into service.
Various acids are used in the pickling process, including hydrochloric acid, sulfuric acid, and phosphoric acid. These acids are effective in dissolving rust and scale, making them essential for chemical cleaning. Depending on the needs, other acids such as citric and acetic acid might also be used.
Inhibitors are added to acid solutions during pickling to protect the base metal of the pipes from being attacked by the acids. These inhibitors are crucial for ensuring the cleaning process does not damage the pipes while effectively removing contaminants.
Neutralizing agents, such as sodium phosphate, are used to neutralize acidic residues after the pickling process. These agents help adjust the pH levels and ensure the pipes are safe from corrosion caused by residual acids.
Regular chemical cleaning is essential for maintaining the performance and longevity of carbon steel pipes. By routinely removing contaminants and preventing corrosion, chemical cleaning helps avoid costly repairs and downtime, ensuring that industrial processes run smoothly and efficiently. Proper handling and storage, along with timely maintenance, are key to maximizing the benefits of chemical cleaning and extending the lifespan of carbon steel pipes.
Carbon steel pipes are metal pipes mainly made of carbon and iron. The carbon content usually ranges from a small amount (0.04%) to a relatively large amount (1.5%). This carbon addition affects the pipes’ mechanical properties, making them stronger, harder, and more wear – resistant. They are very durable and can handle high pressures and temperatures, which is great for tough environments.
There are several types of carbon steel pipes, each for specific uses and conditions:
Carbon steel pipes are widely used in various industries due to their versatility and strong properties. Some common applications are:
In the oil and gas sector, carbon steel pipes are used to transport oil, gas, and other fluids. Their ability to handle high pressures and temperatures makes them perfect for pipelines, refinery systems, and drilling rigs.
In construction, carbon steel pipes are used for structural purposes like building frameworks, scaffolding, and support columns. Their strength and durability ensure the long – term stability of structures.
Power plants use carbon steel pipes to transmit steam, water, and other fluids. They are essential in systems such as boilers, condensers, and heat exchangers, where high – temperature and high – pressure conditions are common.
The automotive and transportation industries, as well as the chemical and petrochemical sectors, utilize carbon steel pipes. In automotive, they are used for vehicle components like exhaust systems, chassis, and suspension parts. In chemical and petrochemical industries, they convey chemicals, acids, and other corrosive substances. The pipes’ wear resistance and ability to handle harsh environments make them suitable for safe and efficient material transport in these sectors.
Carbon steel pipes offer several advantages that make them a top choice in many industries:
Despite their many advantages, carbon steel pipes face challenges, mainly related to corrosion and maintenance.
Chemical cleaning involves using chemical agents to eliminate rust, scale, and organic residues from the inside and outside of carbon steel pipes. This method is essential for maintaining the efficiency and longevity of the pipes, ensuring they remain free from blockages and corrosion that could compromise their structural integrity and performance.
Chemical cleaning plays a critical role in industrial maintenance for several reasons:
Chemical cleaning involves the use of various chemical agents tailored to the specific contaminants and the material of the pipes. The most commonly used chemicals include:
The chemical cleaning process typically involves several stages:
Chemical cleaning is widely used across various industries due to its effectiveness in maintaining pipe integrity and performance:
Pickling primarily restores the pipe’s surface, making it clean, durable, and functional. This process uses dilute acids such as hydrochloric acid (HCl), sulfuric acid (H₂SO₄), or citric acid solutions to dissolve rust and scale on carbon steel pipes. By chemically reacting with iron oxides, pickling forms soluble salts that can be easily flushed away. This method also removes mill scale, a layer of iron oxide formed during manufacturing, which could otherwise lead to corrosion.
Passivation forms a protective oxide film on the surface of carbon steel pipes after cleaning. This film acts as a barrier, reducing the risk of immediate re-rusting and enhancing the pipe’s corrosion resistance,
Hydrochloric acid (HCl) is a strong acid commonly used in the pickling process of carbon steel pipes due to its high reactivity with iron oxides. It effectively dissolves rust and scale quickly and is relatively inexpensive and readily available, making it a popular choice for large-scale industrial cleaning operations.
When using hydrochloric acid, follow strict safety protocols. The acid is highly corrosive and can cause severe burns to the skin and eyes. Ensure proper ventilation to prevent inhaling harmful fumes, and always wear proper protective gear, like gloves, goggles, and protective clothing.
Rust and scale in carbon steel pipes can reduce the internal diameter, restrict fluid flow, and increase energy consumption. Rust can also cause pitting and corrosion, weakening the pipe’s structure and potentially leading to leaks.
Chemical cleaning uses powerful solutions to effectively remove rust, scale, and other contaminants from carbon steel pipes. This process, while effective, presents several risks that necessitate strict adherence to safety protocols to protect personnel, equipment, and the environment.
Acidic cleaning solutions, such as hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), work by dissolving rust and oxides on the surface of carbon steel pipes. The acid reacts with the rust, turning it into soluble salts that can be easily flushed away. In contrast, alkaline rust removers, like sodium hydroxide (NaOH), convert rust into compounds that do not dissolve in water but can be rinsed off mechanically.
Acidic cleaners are highly effective for quickly removing stubborn rust and scale, making them ideal for industrial settings where large-scale and rapid cleaning is required. For example, they are often used in pipelines and heavy machinery maintenance. Alkaline cleaners, on the other hand, are safer and suitable for environments where strong acids are restricted or where the pipes are more sensitive to corrosion. They are also useful for removing organic contaminants like oils and greases, which makes them popular in food processing plants and automotive workshops.
The main advantage of acidic cleaning is its high efficiency in removing rust and scale. However, acidic solutions are highly corrosive, requiring careful handling and the use of inhibitors to protect the base metal. Alkaline cleaning is less corrosive and generally safer to use, but it may require additional steps, such as neutralization after use, and may not be as effective in removing heavy rust and scale as acidic cleaners.
Pickling uses acid solutions to remove rust, scale, and oxide layers from the surface of carbon steel pipes, restoring the pipe’s surface and enhancing its cleanliness. Passivation, performed after pickling or other cleaning processes, forms a protective oxide film on the pipe surface, reducing the risk of re-rusting and enhancing corrosion resistance.
Pickling involves soaking the pipe in an acid solution, which can be circulated inside the pipe to ensure uniform contact with rust and scale. This process requires careful monitoring of the solution’s pH and iron ion concentration. Passivation typically uses chemicals like sodium nitrite to create a passivation solution, which is then circulated inside the pipe until a stable protective layer forms.
Pickling effectively removes surface contaminants, but it can leave the pipe surface more vulnerable to corrosion if not followed by passivation. Passivation provides long-term corrosion protection but requires a clean surface as a starting point. If the pipe is not properly cleaned before passivation, the protective layer may not form correctly.
Acidic cleaning agents are often relatively inexpensive and readily available, making them cost-effective for large-scale cleaning operations. However, the additional costs associated with safety measures, such as proper ventilation and the use of inhibitors, need to be considered. Alkaline cleaners may be more expensive per unit, but they can reduce safety-related costs and environmental impact.
Pickling is necessary for thorough cleaning but can be costly due to the use of acids and the need for precise process control. Passivation adds an extra step and cost but can significantly extend the pipe’s service life, reducing long-term maintenance and replacement costs.
Acidic cleaning is generally faster in removing rust and scale compared to alkaline cleaning. The efficiency of acidic cleaning can be improved by using appropriate inhibitors and optimizing the acid concentration and temperature. Alkaline cleaning efficiency can be enhanced by increasing the solution’s contact time with the pipe and using additives to improve the conversion of rust into insoluble compounds.
The efficiency of pickling can be increased by proper agitation of the acid solution and ensuring uniform contact with the pipe surface. Passivation efficiency can be improved by using the correct chemical composition and concentration of the passivation solution and by controlling the circulation time and temperature.
Industrial pipe cleaning is crucial in multiple sectors to ensure piping systems operate efficiently and last longer. Clean pipes are vital for maintaining optimal flow rates, preventing corrosion, and avoiding costly downtime, especially in the oil and gas industry where they transport crude oil, natural gas, and other hydrocarbons. These pipes often accumulate deposits like paraffin, asphaltenes, and scale, which reduce flow efficiency. Regular cleaning is critical to maintaining pipeline integrity and preventing blockages that could lead to pressure build-up and potential ruptures.
In the power generation sector, clean pipes are essential for the efficient transfer of steam, water, and other fluids. Contaminants like scale and corrosion products can impair heat exchange efficiency and increase the risk of equipment failure. Chemical cleaning maintains the performance of boilers, condensers, and cooling systems, ensuring reliable power generation.
In the chemical and petrochemical industries, pipes often handle corrosive and reactive substances. Deposits and corrosion can compromise the purity of the products and the safety of the process. Effective cleaning ensures that pipes remain free from contaminants, reducing the risk of chemical reactions and maintaining product quality.
Different techniques are used to clean industrial pipes, each suitable for various deposits and pipe materials.
Chemical cleaning uses acids, alkalis, and other chemical agents to dissolve and flush out deposits. This method is highly effective for removing scale, rust, and organic residues. Common chemicals include hydrochloric acid for rust removal and sodium hydroxide for degreasing.
Mechanical cleaning involves physical methods such as brushing, scraping, and pigging. These techniques are effective for removing large, stubborn deposits and are often used in conjunction with chemical cleaning for thorough results.
Hydro jetting uses high-pressure water jets to remove scale, rust, and other deposits from the pipe interior. This method is highly effective for cleaning pipes without the need for chemicals, making it an environmentally friendly option.
A major oil company faced significant flow restrictions in one of its pipelines due to paraffin build-up. The pipeline was chemically cleaned using a combination of solvents and dispersants to dissolve the paraffin. This cleaning process restored the pipeline’s flow capacity and prevented potential blockages that could have led to operational downtime.
In a coal-fired power plant, scale build-up in the boiler tubes reduced heat transfer efficiency and increased fuel consumption. The plant conducted a chemical cleaning operation using hydrochloric acid to dissolve the scale. Post-cleaning inspections showed a significant improvement in heat transfer efficiency and a reduction in fuel costs.
Below are answers to some frequently asked questions:
The best methods for chemically cleaning carbon steel pipes include acidic and alkaline rust removers, passivation, and newer techniques like electrochemical cleaning. Acidic rust removers, such as hydrochloric or citric acid with inhibitors, are fast and effective for heavy rust but require strict pH control. Alkaline removers, like sodium hydroxide solutions, are safer and used to neutralize acidic residues or treat mild rust. Passivation involves circulating sodium nitrite and ammonia to form a protective oxide layer. Electrochemical cleaning applies current to dissolve rust, and hybrid approaches combine mechanical and chemical methods for complex contamination.
To remove rust and scale from carbon steel pipes, chemical cleaning is highly effective. This process typically involves using acidic or alkaline solutions to dissolve or convert the rust and scale. Acidic rust removers, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4), work by chemically dissolving the iron oxides and scale deposits. The procedure involves filling the pipes with the acidic solution, allowing it to react with the rust, and then thoroughly rinsing with water to remove residues. Alkaline rust removers, such as sodium hydroxide (NaOH), convert rust into water-insoluble compounds that can be flushed out. After chemical treatment, it is essential to rinse the pipes thoroughly and neutralize any acidic residues with a mild alkaline solution to prevent further corrosion. Safety protocols must be followed to protect operators and the environment.
During chemical cleaning of carbon steel pipes, several safety protocols must be followed to ensure worker safety and environmental compliance. Personal protective equipment (PPE) is essential; workers should wear chemical-resistant coveralls, certified goggles or full-face shields, nitrile gloves, and NIOSH-approved respirators when necessary. Pre-cleaning preparation involves conducting a thorough hazard assessment to identify contaminants and ensure system isolation to prevent accidental chemical mixing or leaks. Adequate ventilation is also crucial to disperse harmful vapors.
Proper handling and neutralization of chemicals are vital. Compatibility checks should be performed to avoid violent reactions, and neutralization protocols must be followed, using appropriate agents like soda ash for acids and citric acid for bases. Immediate containment of spills using absorbents and proper waste disposal in labeled hazardous containers are necessary steps. Equipment should be protected from chemical exposure, and tools and pipes must be thoroughly cleaned post-use.
Compliance with regulatory guidelines for waste disposal and the use of eco-friendly alternatives whenever possible is essential. Post-cleaning procedures include inspecting the pipes to verify cleanliness and integrity and documenting the entire process. Emergency preparedness involves having an evacuation plan, eyewash stations, and conducting regular training drills. These protocols ensure safety and efficiency during chemical cleaning operations.
Pickling and passivation are essential chemical cleaning processes for carbon steel pipes, offering several benefits crucial for industrial applications.
Pickling involves using acidic solutions to remove impurities like scale, rust, and other contaminants from the surface of carbon steel pipes. This process results in a clean metal surface, enhancing the pipes’ corrosion resistance, which extends their operational lifespan and reduces maintenance needs. Additionally, pickling improves the weldability of carbon steel pipes by creating a smoother surface, facilitating better adhesion during welding and leading to stronger weld joints. Passivation, although less permanent on carbon steel compared to stainless steel, involves applying a temporary corrosion-resistant layer or rust inhibitor to prevent immediate rusting after pickling. This process prepares the surface for further treatments such as coating or painting, ensuring a clean and stable surface. Passivation is particularly beneficial in industries where hygiene is critical, like food and pharmaceutical production, as it ensures the pipes meet stringent quality and safety standards.
Together, pickling and passivation significantly improve the durability, performance, and safety of carbon steel pipes in various industrial settings.
To handle and store hydrochloric acid (HCl) safely, it is essential to follow specific protocols to prevent accidents and ensure compliance with safety regulations. Personal protective equipment (PPE) such as safety goggles, face shields, chemical-resistant gloves, and full-body suits should be worn to protect against splashes and fumes. Work in well-ventilated areas or under a fume hood to minimize inhalation risks, and use acid gas cartridges or respirators if vapor concentrations exceed safe levels.
When handling hydrochloric acid, always add acid to water slowly to prevent exothermic reactions and splashing. Use funnels and pumps to transfer HCl, and ensure that all equipment and containers are made from HCl-resistant materials like PVC, polypropylene, or fiberglass-reinforced plastics.
For storage, use non-metallic containers such as those made from PVC or HDPE to prevent corrosion. Store hydrochloric acid in cool, dry, well-ventilated areas away from direct sunlight, heat sources, and incompatible substances like bases, oxidizers, metals, and organic materials. Containers should be tightly closed, properly labeled with hazard warnings, and placed in spill-proof trays or tubs to contain any leaks.
By adhering to these guidelines, you can safely handle and store hydrochloric acid, ensuring the effective and safe chemical cleaning of carbon steel pipes.
Chemical cleaning is vital for maintaining carbon steel pipes, ensuring their longevity and efficiency. Real-world examples demonstrate its success across various industries:
These examples underscore the importance of choosing the right chemical cleaning method based on the specific needs of carbon steel pipes, ensuring optimal results in both effectiveness and safety.

