Imagine a world where the gleaming brass fixtures in your home or the intricate brass components in your car suddenly lose their luster and strength. This gradual degradation, known as corrosion, can turn valuable assets into liabilities. But what causes brass to corrode, and how can we prevent it? In this beginner-friendly guide, we’ll unravel the mystery behind brass corrosion, exploring the factors that influence its resistance and the mechanisms that lead to its deterioration. From understanding the role of copper and zinc content to examining the impact of environmental conditions, we’ll delve into data-driven insights to equip you with the knowledge to protect and maintain your brass items. Ready to uncover the secrets to extending the life of your brass possessions? Let’s dive in!
Brass is an alloy made from a mixture of copper and zinc. The typical composition of brass is about 66% copper and 34% zinc, though the exact proportions can vary based on the type of brass and its intended use.
Brass is renowned for its versatility and is utilized across various industries due to its durability, corrosion resistance, and attractive appearance. Some common uses of brass include:
Brass is commonly used in plumbing because it resists corrosion and can handle high temperatures.
Brass is ideal for the marine industry because it doesn’t corrode in saltwater, making it perfect for parts like propellers, bearings, and valves.
The automotive industry employs brass for various components, including radiators, hose fittings, and electrical connectors. Brass’s excellent conductivity and resistance to wear make it ideal for these applications, contributing to the Brass is durable, resistant to corrosion, aesthetically pleasing, and easy to work with, making it suitable for many applications.
There are various types of brass, each with different properties based on their specific copper and zinc ratios:
Alpha brass contains between 35% and 45% zinc. It is known for its excellent ductility and corrosion resistance, making it suitable for architectural and decorative applications.
Alpha – beta brass, with approximately 55 – 60% copper and 35 – 45% zinc, offers higher strength and hardness. This type of brass is commonly used in fittings, valves, and other components requiring enhanced mechanical properties.
Beta brass, which has a higher zinc content, is primarily used for die – casting due to its fluidity and low melting point. It is suitable for applications where complex shapes and fine details are required.
Understanding the composition, characteristics, and applications of brass helps in selecting the appropriate type for specific uses, ensuring optimal performance and longevity.
Corrosion is the slow deterioration of materials, usually metals, due to chemical reactions with their surroundings. For brass, an alloy primarily composed of copper and zinc, corrosion mechanisms can vary based on environmental conditions and the specific composition of the alloy.
Unlike iron or steel, brass doesn’t rust; it experiences other types of corrosion. These processes can affect the material’s appearance and structural integrity over time.
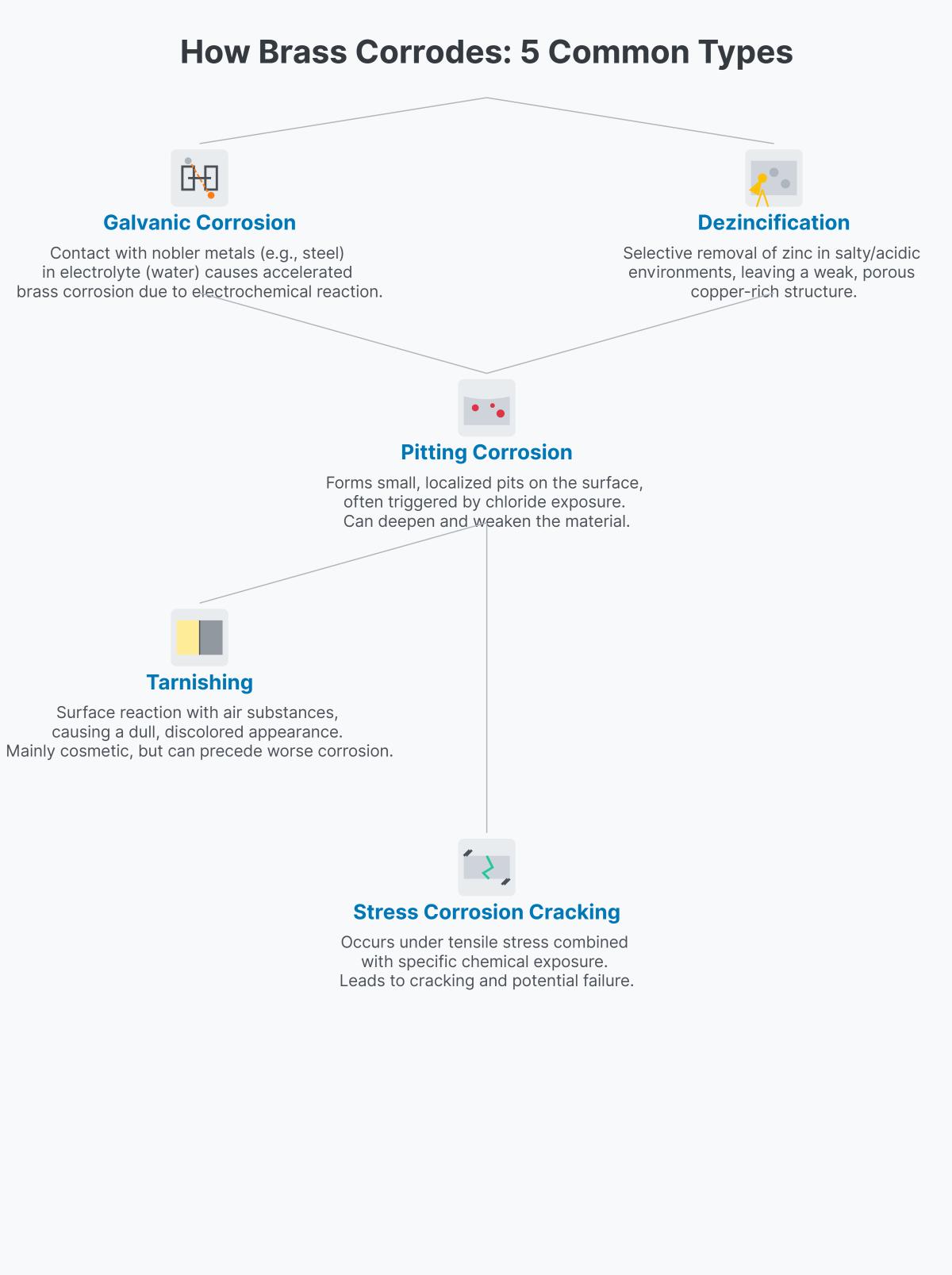
Tarnishing is the most frequent and least damaging type of corrosion in brass. It occurs when the surface of brass reacts with oxygen in the air, forming a thin layer of oxide. This results in a dull, discolored appearance but does not significantly impact the metal’s strength or functionality. Tarnishing can be removed through polishing and cleaning.
Dezincification is a more serious form of corrosion where zinc is selectively leached from the brass alloy. This typically occurs in environments with high levels of chlorides or other aggressive agents. The result is a porous, copper-rich structure that is significantly weaker and more prone to failure. Dezincification can lead to leaks and mechanical failures, particularly in plumbing and marine applications.
Stress Corrosion Cracking (SCC) occurs when brass cracks due to the combination of stress and a corrosive environment. Chemicals such as ammonia or chlorides can induce SCC, leading to sudden and unexpected failures. This type of corrosion is especially dangerous because it can occur without significant prior warning signs.
Galvanic corrosion occurs when brass comes into electrical contact with a more noble metal, such as copper or stainless steel, in the presence of an electrolyte (moist environment). The electrochemical interaction accelerates the corrosion of the less noble metal (brass), leading to rapid degradation. This type of corrosion is common in systems where different metals are used together.
Pitting corrosion involves the formation of small, localized holes or pits on the surface of brass. These pits can penetrate deep into the metal, leading to significant material loss and potential failure. Pitting is hard to detect early on but can cause significant damage over time.
As previously mentioned, dezincification is the selective leaching of zinc from brass. It leaves behind a weakened, porous structure. This form of corrosion is prevalent in environments with high chloride content, such as seawater or chlorinated water systems.
SCC in brass is induced by tensile stress in combination with a corrosive environment. The presence of ammonia or chlorides can initiate cracking, which can propagate quickly, leading to sudden failure of the brass component.
Understanding these types of corrosion and their mechanisms helps in selecting the appropriate brass alloys and implementing preventive measures to enhance the durability and longevity of brass components in various applications.
Understanding the factors that influence the corrosion resistance of brass is essential for selecting the right type of brass for specific applications and ensuring its longevity. Several key factors play a role in how well brass can withstand corrosion.
The copper content in brass significantly impacts its corrosion resistance. When brass is exposed to the atmosphere, copper forms a stable oxide layer that protects against further oxidation and corrosion.
Zinc content affects the corrosion resistance of brass; although it enhances mechanical properties, higher zinc levels can increase susceptibility to dezincification. Dezincification is a type of corrosion where zinc is selectively leached out, leaving behind a porous, weakened structure. This is particularly problematic in water-rich environments, such as plumbing systems and marine applications.
Moisture in the environment can accelerate the corrosion of brass. High humidity levels facilitate electrochemical reactions on the brass surface, leading to faster corrosion rates. This is particularly relevant in marine environments and other areas with high levels of atmospheric moisture.
Temperature is another critical factor. Higher temperatures increase the rate of chemical reactions, including those that cause corrosion. Therefore, brass components exposed to elevated temperatures may corrode more quickly than those in cooler environments.
Pollutants like sulfur dioxide and ammonia can greatly affect brass’s corrosion resistance. These gases can react with the brass surface to form corrosive acids, leading to accelerated corrosion. This is a common issue in industrial areas where these pollutants are prevalent.
Acidic environments with chlorides and acetates can increase corrosion rates in brass. These chemicals can weaken the protective oxide layer on brass, making it more susceptible to further corrosion.
Exposure to sulfur compounds can cause tarnishing, which is the formation of a dark layer of copper sulfide on the brass surface. While tarnishing is primarily a cosmetic issue, it can indicate the presence of corrosive sulfur compounds in the environment.
Mechanical stress can lead to stress corrosion cracking (SCC) in brass. SCC occurs when tensile stress and a corrosive environment cause the brass to crack. This type of corrosion is particularly dangerous because it can lead to sudden and unexpected failures of brass components.
Galvanic corrosion occurs when brass comes into contact with a more noble metal, such as silver or gold, in the presence of an electrolyte (such as water). This electrochemical interaction accelerates the corrosion of the less noble metal (brass), leading to rapid degradation.
Protective coatings can greatly improve brass’s corrosion resistance. Coatings such as zinc, nickel, and polymer create a barrier that prevents corrosive elements from reaching the brass surface. Additionally, incorporating elements like tin, nickel, or arsenic into the brass alloy can prevent dezincification and form protective layers.
Certain specialized brass alloys are designed to offer improved resistance to corrosion. For example, naval brass, which includes a small percentage of tin, provides enhanced corrosion resistance in marine environments. These specialized alloys are crucial for applications where standard brass may not offer sufficient durability.
Standards are essential for preventing corrosion in brass, as they set guidelines and requirements that ensure the quality, safety, and performance of materials and products. Following these standards helps manufacturers create durable brass components that can endure different environments and conditions.
The American Society for Testing and Materials (ASTM) has developed several standards specifically addressing brass and its corrosion resistance. These standards provide detailed procedures for testing, material composition, and performance criteria.
The International Organization for Standardization (ISO) also provides comprehensive standards for brass corrosion testing and material specifications.
Compliance with regulatory standards is essential for ensuring that brass components are safe and suitable for their intended applications, particularly in critical sectors such as plumbing and potable water systems.
Recent regulations, such as those outlined in the Safe Drinking Water Act, mandate the use of lead-free materials in plumbing systems to protect public health. Lead-free brass, using elements like bismuth or silicon instead of lead, must meet these regulations.
Adhering to established standards for brass corrosion resistance offers several significant benefits:
By following these standards, manufacturers can produce high-quality brass components that offer superior corrosion resistance, ensuring their suitability for various demanding applications.
Brass is widely used in plumbing and water systems. Its corrosion resistance ensures long-lasting use, preventing leaks and water contamination, which is why faucets, valves, and pipe fittings are commonly made from brass. For example, brass faucets can maintain their functionality and appearance for many years, even with constant exposure to water.
Brass’s high conductivity and corrosion resistance make it perfect for electrical connectors, terminals, and switches, ensuring efficient electricity transfer and durability. This reliability makes it a staple in both household and industrial electrical setups.
In machinery and engineering, brass’s low friction properties are invaluable. It is used for moving parts like gears and bearings that require materials capable of reducing wear and tear, contributing to the smooth operation of machinery.
Brass’s warm, golden appearance makes it a favorite for decorative items. From sculptures to jewelry, brass adds an elegant touch. It can be easily shaped into various forms, allowing artists and designers to create intricate and beautiful pieces.
Brass is commonly used in musical instruments like trumpets and saxophones due to its ability to produce rich and resonant sounds. Musicians rely on the unique qualities of brass to create beautiful music.
Below are answers to some frequently asked questions:
Galvanic corrosion occurs when brass comes into contact with a more noble metal, such as stainless steel or silver, in the presence of an electrolyte like water. An electrochemical reaction ensues, causing the brass to corrode at an accelerated rate. This interaction results in material degradation over time.
Pitting corrosion is characterized by small, localized pits on the brass surface. Specifically, chloride exposure often triggers pitting corrosion. Over time, these pits can deepen, significantly weakening the brass material.
In salty or acidic environments, zinc is selectively removed from the brass alloy. This results in a weaker, porous copper-rich structure. Dezincification can compromise the mechanical integrity of brass components, making them more prone to failure.
Tarnishing is a surface-level issue that occurs when brass reacts with substances in the air, leading to a dull, discolored appearance. Though it’s mainly a cosmetic issue, unaddressed tarnishing can signal more serious corrosion ahead.
Specifically, when brass is under tensile stress and exposed to certain chemicals, it can develop stress corrosion cracking. This leads to cracking and potentially the failure of the brass component, often without much prior warning.
The corrosion resistance of brass, an alloy composed mainly of copper and zinc, is influenced by several factors. The copper content in brass enhances its corrosion resistance because it forms a protective patina that shields the metal from further damage. On the other hand, high zinc content can make brass more susceptible to dezincification, where zinc is selectively removed, especially in harsh environments like saltwater.
Environmental conditions also play a significant role. Moisture, saltwater, chlorides, and high acidity levels can accelerate corrosion. High temperatures can further speed up this process. Exposure to sulfur compounds can cause tarnishing, while ammonia can lead to stress corrosion cracking under mechanical stress.
To improve brass durability, protective coatings and the addition of alloying elements like tin or lead can be effective. Regular maintenance practices also help in preventing corrosion and maintaining brass’s longevity.
Dezincification is a specific type of corrosion that affects brass, which is an alloy primarily composed of copper and zinc. During dezincification, zinc is selectively leached out from the brass, leaving behind a porous, weakened copper-rich structure. This process typically occurs in environments where the brass is exposed to slightly acidic or alkaline conditions, chloride ions (such as in seawater), carbon dioxide, or oxygen.
The two main types of dezincification are plug-type, which creates localized deep pits or holes, and layer-type, which uniformly reduces the wall thickness of the brass component. The visible signs of dezincification include a color change from the typical yellow of brass to a pink or red hue due to the higher copper content. To prevent dezincification, using dezincification-resistant (DZR) brass or alloys with lower zinc content, as well as maintaining regular maintenance and avoiding exposure to corrosive environments, is recommended.
To prevent stress corrosion cracking (SCC) in brass, several strategies can be employed. Firstly, selecting brass alloys with low zinc content or those containing nickel, like nickel silver, can enhance resistance to SCC. Aluminum brass and bronze are also good alternatives. Stress relief techniques, such as stress relief heat treatment, help reduce internal stresses in brass components. This process typically involves heating the brass to 250°C to 300°C for about half to one hour. Additionally, avoiding cold working processes that introduce stresses can minimize SCC risks. Managing the environment is crucial; reducing exposure to ammonia and preventing moisture condensation are effective measures. Proper assembly practices, such as avoiding over-tightening and using appropriate tools, also help prevent localized stresses that can lead to SCC.
To maintain the durability of brass, several best practices should be followed. Regular cleaning is essential; use a soft, dry cloth to remove dust and dirt, and for deeper cleaning, a mild soap solution with warm water is effective. Ensure thorough rinsing and drying to avoid water spots. Applying protective coatings, such as lacquer or wax, can shield brass from air and moisture, preserving its shine and color. Store brass items in a cool, dry place, away from direct sunlight and moisture, and use soft cloths or felt for wrapping to prevent scratches. Polishing with commercial brass polishes or homemade solutions like baking soda and lemon juice helps remove tarnish. In corrosive environments, consider using corrosion inhibitors or methods like cathodic protection. Avoid harsh chemicals and abrasive materials, and handle brass items carefully to prevent damage. Regular inspections can help detect early signs of corrosion, allowing for timely maintenance.
Standards compliance is important for brass corrosion prevention because it ensures material quality and consistency. Standards like EN 12165 and ASTM B887 specify the composition of dezincification – resistant brass, guaranteeing consistent performance. It also reduces the risk of dezincification by guiding the selection of appropriate alloys. Compliance facilitates reliable engineering decisions, helping engineers choose the right materials and design systems that perform well under various conditions. Moreover, it supports environmental sustainability by reducing waste and energy consumption. Lastly, it enhances product longevity and performance, allowing brass components to withstand harsh environments over time.

