Imagine purchasing a sleek stainless steel appliance for your kitchen, only to find unsightly rust spots appearing over time. You might wonder, “How can something called ‘stainless’ steel actually corrode?” Contrary to popular belief, stainless steel is not entirely immune to corrosion. While it is highly resistant, certain environmental factors and improper maintenance can lead to its deterioration. In this article, we’ll explore the common causes of stainless steel corrosion, from exposure to harsh chemicals to mechanical damage. More importantly, we’ll provide practical solutions to prevent it, such as applying protective coatings and selecting the right grade of stainless steel for your needs. By understanding these concepts, you can ensure the longevity and pristine condition of your stainless steel items. So, what measures can you take to protect your investment from corrosion? Let’s dive in to find out.
Stainless steel is an alloy made mainly of iron, with at least 10.5% chromium, which provides its notable resistance to rust and staining. This chromium content is crucial as it forms a thin, passive layer of chromium oxide on the surface, serving as a protective barrier that prevents further oxidation and corrosion.
Corrosion is the gradual degradation of materials, usually metals, due to chemical reactions with their environment. For stainless steel, corrosion typically occurs when the passive layer is damaged or compromised, exposing the underlying metal to corrosive elements.
The passive layer on stainless steel is self-healing; when damaged, it can spontaneously reform in the presence of oxygen, maintaining the metal’s resistance to corrosion. However, if the environment lacks sufficient oxygen or is highly aggressive (e.g., high chloride concentrations), the passive layer may not reform effectively, leading to corrosion.
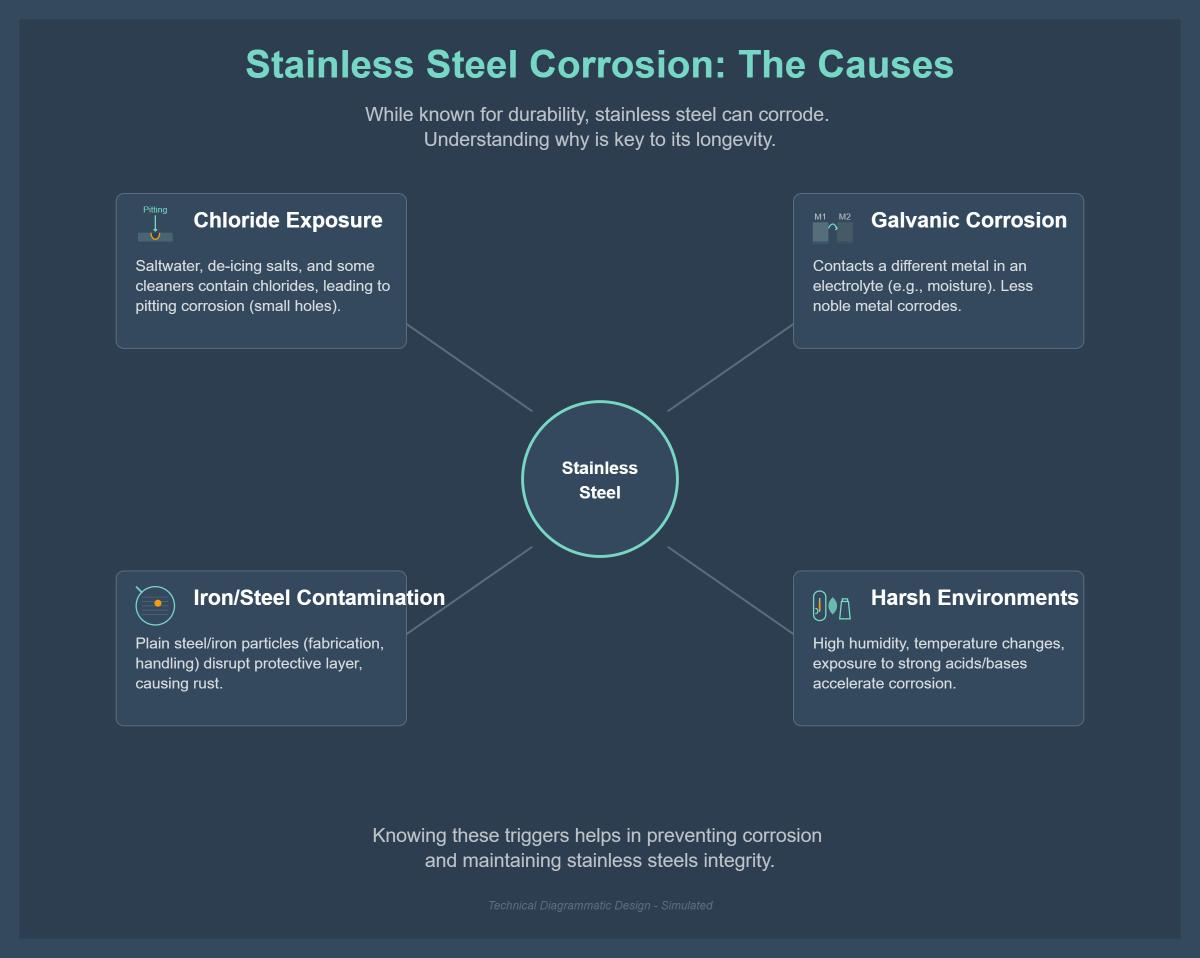
Despite its resilience, stainless steel can corrode under certain conditions, such as high chloride exposure, low oxygen levels, high humidity, and contact with more anodic metals.
Stainless steel can suffer from various forms of corrosion, including:
Stainless steel is an alloy made primarily of iron, known for its exceptional resistance to corrosion and staining. This unique property is primarily due to its chromium content, which must be at least 10.5% for the steel to be classified as stainless.
Stainless steel’s composition typically includes:
Stainless steel possesses several key properties that make it a preferred material in various applications:
Stainless steel can be categorized into several types based on its microstructure and alloying elements:
Stainless steel’s versatility allows it to be used in a wide range of applications, including:
Stainless steel often corrodes when exposed to strong chlorides, like those in seawater or salty environments. Chlorides can break through the protective chromium oxide layer on stainless steel, causing pitting corrosion. This results in small pits or holes on the surface, which compromise the material’s integrity. To prevent pitting corrosion, it’s advisable to use chloride-resistant stainless steel grades like 316 or apply protective coatings that shield the metal from chlorides.
Bimetallic or galvanic corrosion occurs when stainless steel is in contact with a different type of metal. This contact can create a galvanic cell, causing the less noble metal to corrode at an accelerated rate. Preventing galvanic corrosion involves using similar metals to avoid the creation of galvanic cells or applying insulating coatings to prevent electron flow between dissimilar metals.
Particles from plain iron or steel can transfer to stainless steel surfaces, especially during manufacturing or maintenance processes. These particles can disrupt the protective oxide layer, leading to rust and corrosion. Proper cleaning and maintenance of equipment can prevent the transfer of plain iron or steel particles to stainless steel surfaces, maintaining its corrosion resistance.
General corrosion happens when chemicals or acids uniformly attack stainless steel, degrading its entire surface. Selecting the appropriate stainless steel grade that is resistant to specific chemicals or acids is crucial in preventing general corrosion.
Crevice corrosion usually occurs in tight spaces where oxygen can’t reach, like under gaskets, washers, or deposits. These confined spaces allow chlorides to concentrate and initiate corrosion. To prevent crevice corrosion, designs should avoid creating tight crevices, and more resistant stainless steel grades should be used. Sealing crevices effectively can also help prevent this type of corrosion.
Stress corrosion cracking (SCC) happens due to a combination of stress, high temperatures, and exposure to corrosive substances like chlorides, causing cracks that weaken the material. Prevention strategies include selecting alloys that are less susceptible to SCC and avoiding conditions that impose high stress on the material.
Intergranular corrosion occurs at the grain boundaries of stainless steel, often due to chromium carbide formation during welding or improper heat treatment. This type of corrosion can weaken the metal structure significantly. Using low-carbon stainless steel grades or proper heat treatment techniques can minimize the risk of intergranular corrosion.
Choosing the right stainless steel grade for the specific environment is crucial. For example, using grade 316 in chloride-rich environments provides better resistance to pitting corrosion.
Applying protective coatings can prevent contact with harmful chemicals, while insulators can separate dissimilar metals to prevent galvanic corrosion.
Regular cleaning and proper maintenance of equipment can prevent the transfer of particles from other metals, maintaining the stainless steel’s protective oxide layer.
Designing components to avoid tight crevices and stress concentrations can reduce the risk of crevice corrosion and stress corrosion cracking.
Managing factors such as temperature, humidity, and chemical exposure can significantly reduce the risks of corrosion.
Pitting corrosion causes small pits or holes to form on the surface of stainless steel. This type of corrosion is primarily caused by exposure to chloride ions, which are commonly found in environments such as seawater and de-icing salts.
Crevice corrosion occurs in confined spaces where oxygen cannot reach, allowing corrosive substances like chlorides to accumulate. This type of corrosion is commonly found in areas such as under gaskets, washers, and deposits.
General corrosion uniformly attacks the surface of stainless steel, typically due to acidic environments, leading to
Stress corrosion cracking (SCC) is a severe form of corrosion that involves the combined effects of tensile stress, high temperatures, and exposure to corrosive substances like chlorides. SCC can lead to rapid cracking and failure of stainless steel components.
Intergranular attack occurs at the grain boundaries of stainless steel, often due to improper heat treatment or welding processes. This type of corrosion can significantly weaken the metal structure.
Galvanic corrosion occurs when two dissimilar metals are in contact with an electrolyte, leading to accelerated corrosion of one of the metals. This type of corrosion is common in environments where stainless steel is used alongside other metals.
Protective coatings are a highly effective way to prevent stainless steel from corroding, acting as barriers against moisture, chemicals, and salts. Epoxy-based coatings provide excellent protection against chemical exposure and are widely used in industrial environments, while polyurethane coatings offer superior resistance to abrasion and chemical attacks, making them suitable for harsh conditions. Powder coatings are durable and provide a uniform protective layer, ideal for both aesthetic and functional purposes.
Surface treatments enhance the natural corrosion resistance of stainless steel by improving the protective oxide layer or adding additional protective layers. Passivation involves treating the stainless steel with an acid solution to remove contaminants and enhance the formation of the protective chromium oxide layer. Electropolishing smooths and polishes the surface, reducing the risk of corrosion by eliminating surface irregularities. Although not commonly used for stainless steel, anodizing can provide an additional protective layer for certain applications.
Choosing the right stainless steel grade for the specific environment and application is crucial in preventing corrosion. 304 Stainless Steel is the most widely used grade, suitable for general-purpose applications with moderate corrosion resistance, while 316 Stainless Steel, known for its higher resistance to chlorides and other corrosive substances, is ideal for marine environments and chemical exposure. Duplex stainless steels combine austenitic and ferritic properties, offering superior strength and resistance to stress corrosion cracking.
Managing the environmental conditions in which stainless steel is used can significantly reduce the risk of corrosion. Minimizing exposure to moisture through proper drainage systems and using water-resistant coatings can prevent corrosion. Avoiding extreme temperatures, particularly in welding processes, helps maintain the integrity of the protective oxide layer. Limiting exposure to aggressive chemicals and using appropriate containment and ventilation systems can protect stainless steel from chemical attacks.
Regular maintenance and cleaning are essential to prevent the buildup of contaminants that can lead to corrosion. Use non-abrasive, mild detergents to clean stainless steel surfaces, ensuring that the protective oxide layer is not damaged. Refrain from using abrasive cleaning materials that can scratch the surface and compromise the protective layer. Regularly inspect stainless steel components for signs of corrosion and perform necessary maintenance to address any issues promptly.
Welding can weaken the protective oxide layer, making stainless steel more susceptible to corrosion. Ensure weld sites are cleaned before and after welding to remove contaminants, apply post-weld treatments like passivation to restore the protective layer, and use controlled heat input to avoid excessive heating that can damage the protective layer.
Regular cleaning is crucial for preserving the quality and look of stainless steel surfaces. Use mild soap and warm water to clean stainless steel, avoiding harsh chemicals or abrasive materials. Rinse thoroughly and dry immediately to prevent water spots and corrosion.
Regularly check for signs of corrosion like discoloration or rust, and address any issues promptly. Use passivation treatments to boost corrosion resistance by cleaning and applying an acid bath to remove contaminants.
Apply protective coatings like epoxy or polyurethane to shield stainless steel from moisture and contaminants. Also, avoid chlorides and rinse off any exposure with fresh water.
Use soft cloths or non-abrasive sponges for cleaning stainless steel surfaces to avoid scratching the protective layer. When cleaning with chemicals, wear safety gear such as gloves and goggles to protect against splashes and ensure safe handling.
Minimize exposure to high humidity and extreme temperatures, which can accelerate corrosion. Use dehumidifiers and proper ventilation to control the environment around stainless steel components. Limit the exposure of stainless steel to aggressive chemicals by using containment systems and proper ventilation. This helps protect the metal from chemical attacks that can lead to corrosion.
Stainless steel is extensively used in the automotive industry due to its strength, durability, and excellent corrosion resistance. Key applications include:
In the healthcare industry, stainless steel is favored for its hygiene, ease of cleaning, and resistance to corrosion. Common applications include:
Stainless steel is widely used in architecture and construction for its strength, flexibility, and aesthetic qualities. Notable applications include:
The aerospace industry relies on stainless steel for its ability to withstand extreme temperatures and its high strength-to-weight ratio. Applications include:
In the food and beverage industry, stainless steel is essential for its hygiene and resistance to corrosion. Key applications include:
Despite its many advantages, stainless steel can face several challenges in various applications:
Below are answers to some frequently asked questions:
Stainless steel, known for its corrosion resistance, can still corrode under certain conditions. The primary causes include exposure to chlorides, such as those found in saltwater or some cleaning agents, which can lead to pitting corrosion. Galvanic corrosion can occur when stainless steel is in contact with other metals in the presence of an electrolyte, causing the less noble metal to corrode faster. Contamination from plain steel or iron, often occurring during fabrication, can disrupt the protective oxide layer, leading to rust. Additionally, environmental factors like high humidity, temperature changes, and exposure to acids or bases can accelerate corrosion. Understanding these causes is essential for maintaining stainless steel’s integrity and longevity.
To prevent stainless steel corrosion, several effective strategies can be employed. Regular cleaning with mild detergents and soft cloths helps maintain the protective oxide layer, which is crucial for corrosion resistance. Passivation, especially after welding, restores this protective layer by removing contaminants that can lead to corrosion. Avoiding abrasives and free iron contamination is also important; use plastic scouring pads instead of steel wool to prevent damaging the stainless steel surface.
Proper storage is essential to prevent galvanic corrosion; stainless steel should be stored in dry conditions away from other metals. Applying protective coatings, such as rust-resistant layers, can enhance protection against environmental factors like chlorides and humidity. Additionally, selecting the appropriate grade of stainless steel for specific environments is key; for instance, type 316 stainless steel offers better resistance to chlorides than type 304.
By implementing these strategies, users can significantly reduce the risk of stainless steel corrosion and extend the material’s lifespan in various applications.
Stainless steel is categorized into several main types, each with distinct properties that suit different applications and environments. The primary types include Austenitic, Ferritic, Martensitic, and Duplex stainless steels.
Austenitic stainless steels, such as Grade 304 and Grade 316, are known for their high corrosion resistance and good tensile strength. Grade 304 contains 18% chromium and 8% nickel, making it suitable for everyday products, while Grade 316 includes molybdenum, which enhances resistance to chloride corrosion, ideal for marine environments. Lower carbon versions, like 304L and 316L, are designed to reduce carbide precipitation during welding, preventing corrosion in welded areas.
Ferritic stainless steels, including Grade 430 and Grade 434, offer good ductility and resistance to atmospheric corrosion but are generally less strong than austenitic steels.
Martensitic stainless steels, such as Grade 420 and Grade 440, are known for their high tensile strength and hardness. Grade 420 is often used in cutlery and surgical instruments, while Grade 440 can be hardened to higher levels, making it suitable for razor blades.
Duplex stainless steels, including Standard Duplex (e.g., Grade 2205) and Super Duplex (e.g., Grade 2507), provide a balance of strength and corrosion resistance, commonly used in pipelines and demanding environments. Lean Duplex offers a cost-effective option for less demanding applications.
Understanding these different grades helps in selecting the appropriate stainless steel for specific applications and environments, thereby preventing corrosion and ensuring durability.
Protective coatings are vital in preventing stainless steel corrosion by forming a barrier between the metal surface and corrosive elements such as moisture, oxygen, and salts. This barrier reduces the corrosion rate and significantly extends the lifespan of the metal. Some coatings, like those containing zinc, offer sacrificial protection by corroding preferentially, thereby safeguarding the underlying metal. Additionally, many coatings are designed to resist chemical reactions, making it difficult for corrosive substances to penetrate. Advanced coatings may even have self-healing properties to repair minor damages autonomously, ensuring continuous protection.
The frequency of maintaining stainless steel to prevent corrosion depends on the environmental conditions and the specific application. In general indoor conditions, stainless steel should be maintained biannually, involving cleaning with soap and water and applying a protective lubricant. For outdoor or harsh environments, such as coastal or industrial areas with high humidity or chemical exposure, maintenance should be performed quarterly to remove contaminants and prevent pitting corrosion. In hygienic applications like food handling or medical environments, daily or after-use cleaning is necessary to ensure cleanliness and prevent contamination. Regular maintenance practices include using non-abrasive tools, cleaning with the grain, avoiding chloride cleaners, and inspecting for signs of corrosion.
Regular maintenance plays a vital role in preventing corrosion of stainless steel. Even though stainless steel is known for its resistance to corrosion, it can still be susceptible under certain conditions. Regular maintenance involves several key practices:
By implementing these maintenance practices, the lifespan of stainless steel can be extended, and overall maintenance costs can be reduced, ensuring safety and efficiency in its applications.

