Have you ever noticed mysterious rust spots on your shiny metal fixtures after a thorough cleaning session? You might be surprised to learn that bleach, a common household cleaner, could be the culprit. While bleach is renowned for its disinfecting power, it can have unintended and often damaging effects on various metal surfaces. In this article, we’ll delve into how bleach interacts with metals, which types are most susceptible to damage, and the chemical reactions at play. Moreover, we’ll explore practical strategies for protecting your metal surfaces from bleach-induced corrosion and suggest safer alternatives for cleaning. Ready to safeguard your metal belongings? Let’s dive in and uncover the secrets to maintaining their luster and longevity.
Bleach, which primarily consists of sodium hypochlorite, is known for its strong cleaning and disinfecting properties. However, when bleach comes into contact with metal surfaces, its strong oxidizing properties can lead to several adverse effects.
Understanding these interactions and adopting appropriate safe practices can significantly reduce the risk of damage when using bleach on metal surfaces. By following these guidelines, it is possible to maintain the integrity and longevity of metal materials while achieving effective cleaning results.
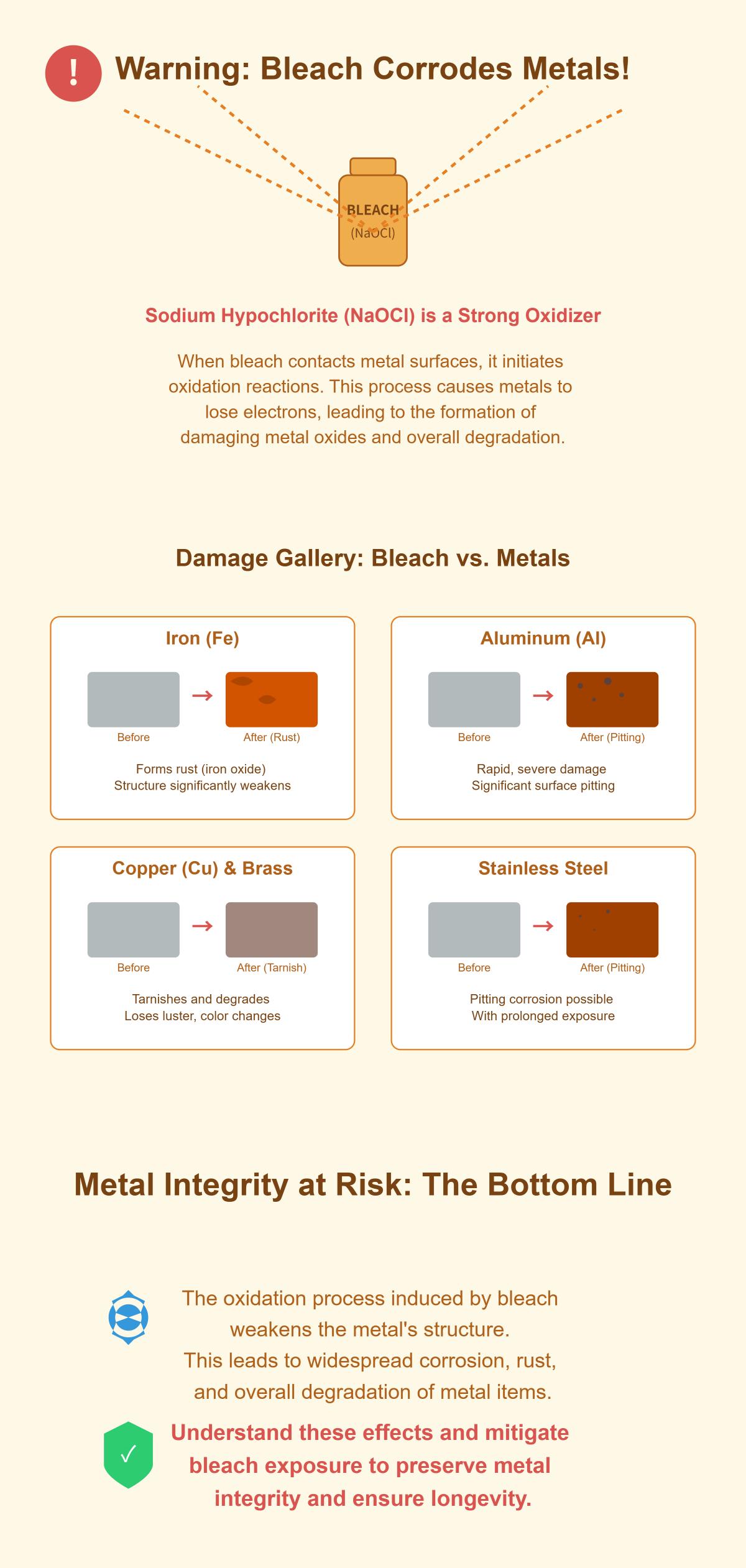
Bleach, primarily composed of sodium hypochlorite (NaOCl), is widely used for its potent disinfecting and cleaning capabilities. Although bleach is effective for removing stains and sanitizing surfaces, it can significantly damage metal surfaces. Understanding how bleach, a strong oxidizing agent, reacts with metals can help mitigate these adverse effects.
When bleach comes into contact with metal surfaces, it initiates oxidation reactions that result in the formation of metal oxides. The general chemical reaction can be represented as follows:
Metal+NaOCl→Metal Oxide+Other Products
For instance, when iron interacts with bleach, iron oxide (Fe₂O₃) forms, commonly known as rust. The presence of moisture accelerates this process, further weakening the metal structure.
To mitigate the corrosive effects of bleach on metal surfaces, dilution is a key strategy. Diluting bleach with water reduces the concentration of sodium hypochlorite, thereby lessening its aggressive impact. A common dilution ratio is ⅓ cup of bleach per gallon of water. Additionally, limiting the exposure time of bleach on metal surfaces can prevent severe damage. It is crucial to rinse the metal thoroughly with clean water after bleach application to remove any residues.
Using protective coatings like clear lacquers or anti-corrosion sprays can protect metal surfaces from bleach damage. These coatings create a barrier that prevents direct contact between the bleach and the metal, preserving the metal’s integrity.
For metals that are particularly susceptible to bleach damage, such as aluminum and copper, using milder cleaning agents is recommended. Alternatives like vinegar, baking soda, or specialized metal cleaners can effectively clean these surfaces without causing the detrimental reactions associated with bleach.
Bleach is a potent and corrosive chemical that requires careful handling. It can cause skin and eye irritation and respiratory distress. Additionally, bleach is incompatible with several chemicals, including acids, alcohols, and ammonia-containing compounds, which can produce toxic gases when mixed. Always use proper ventilation and protective gear when handling bleach to ensure safety and avoid hazardous reactions.
Stainless steel, renowned for its corrosion resistance, is still vulnerable to damage from bleach. Extended exposure to bleach can damage the protective chromium oxide layer, causing pitting, discoloration, and eventual corrosion. Even diluted bleach solutions can be harmful over time, so it’s essential to limit exposure and rinse thoroughly after cleaning.
Bleach can severely damage aluminum’s integrity and appearance. When bleach comes into contact with aluminum, it can cause rapid and severe corrosion, leading to pitting and surface degradation. This reaction is aggressive enough that it’s generally recommended to avoid using bleach on aluminum surfaces altogether.
Bleach speeds up rusting in iron and steel by promoting oxidation, forming rust that weakens and damages the metal. This accelerated rusting process can quickly compromise the structural integrity of these metals, making it crucial to prevent prolonged exposure and ensure thorough rinsing if bleach is used.
When bleach reacts with these metals, it forms copper oxides that can greatly alter their appearance and surface quality. Copper and brass are prone to tarnishing and discoloration after even brief contact with bleach, so it’s advisable to use alternative cleaning agents for these metals.
Bleach reacts with the zinc coating, forming zinc oxide that dulls the surface and reduces its protective qualities. Over time, this reaction can lead to corrosion and diminished durability of the galvanized steel.
Corrosion is a natural process that gradually degrades metals through chemical reactions with their environment. This deterioration affects the metal’s mechanical properties, appearance, and functionality. Recognizing and preventing corrosion is crucial for maintaining the longevity and integrity of metal surfaces, especially when they are exposed to oxidizing agents like bleach.
One effective way to prevent corrosion is to dilute bleach before applying it to metal surfaces. A common dilution ratio is ⅓ cup of bleach per gallon of water. This reduces the concentration of sodium hypochlorite, minimizing its corrosive impact.
Minimize the contact time between bleach and metal surfaces. Apply bleach only for the necessary duration, then promptly rinse the metal with clean water to remove any residues. This practice helps prevent prolonged exposure that can lead to corrosion.
Applying protective coatings can protect metal surfaces from bleach. Options include lacquers, waxes, and anti-corrosive paints.
Consider using gentler cleaning agents that are less corrosive than bleach. Some alternatives include:
Regularly inspect metal surfaces for signs of corrosion. Early detection allows for prompt action to mitigate damage. Look for changes in color, texture, and structural integrity.
Ensure good ventilation when using bleach or other cleaning agents. Proper airflow helps disperse harmful fumes that can contribute to corrosion.
By adopting these preventive measures, you can effectively protect metal surfaces from the corrosive effects of bleach, ensuring their longevity and durability.
Adopting safe usage practices is crucial to minimize exposure and prevent damage to metal surfaces from bleach. Here are some key practices to follow:
Dilution, such as using a 1:10 or 1:50 water-to-bleach ratio, lowers the concentration of sodium hypochlorite, making the solution less aggressive on metal surfaces.
Minimizing the duration that bleach remains on metal surfaces is essential. Ensure that bleach does not stay on the surface for more than a few minutes. Prolonged exposure can cause severe rust and damage to the metal.
After using bleach, it is imperative to rinse the metal surface thoroughly with clean water. This step removes any residual bleach, preventing ongoing chemical reactions that could cause further damage. Rinsing also helps neutralize any remaining oxidizing agents.
Using milder cleaning agents can effectively clean metal surfaces without the risks associated with bleach. Several alternatives are available that offer safe and efficient cleaning:
Applying protective coatings to metal surfaces can create a barrier against bleach and other corrosive agents, helping to maintain the metal’s appearance and structural integrity.
Stainless steel polishes not only enhance the appearance of the surface but also provide a protective layer that can help resist damage from bleach and other chemicals.
For surfaces that come into contact with food, applying food-safe mineral oils can create a protective barrier against spills and contaminants, including bleach.
Clear lacquers or waxes can be applied to metal surfaces to shield them from bleach and other harsh chemicals. These coatings protect the metal’s look and strength.
Implementing best practices when using bleach around metal surfaces can further prevent damage and ensure safety.
Before using bleach in areas where metal fixtures are present, cover these fixtures with plastic or other protective materials to prevent accidental exposure.
Keep the area well-ventilated when using bleach. Proper ventilation helps disperse fumes that could otherwise settle on metal surfaces and cause corrosion.
Perform a spot test by applying bleach to a small, inconspicuous area of the metal surface before wider application. This test helps assess potential damage and ensures that the bleach will not adversely affect the metal.
Bleach is often too harsh for metal surfaces, causing corrosion, discoloration, and damage. Fortunately, there are several effective alternatives that can clean metal without these adverse effects.
Vinegar’s acidity dissolves mineral deposits, while baking soda acts as a mild abrasive to scrub away dirt without scratching. Together, they effectively remove stains and polish metal surfaces. Create a paste using equal parts vinegar and baking soda, apply it to the metal surface, scrub gently with a soft brush, and rinse thoroughly.
Lemon juice is another natural cleaning agent that works well on metal surfaces, especially copper and brass. Its citric acid content helps to break down tarnish and restore the metal’s shine. Apply lemon juice directly or mixed with salt for extra scrubbing power. Wipe clean with a damp cloth.
Hydrogen peroxide is a safer disinfectant compared to bleach. It effectively sanitizes metal surfaces without causing corrosion. Spray hydrogen peroxide onto the metal surface, let it sit for a few minutes, and then wipe clean with a cloth. It is particularly useful for stainless steel surfaces where maintaining the aesthetic is crucial.
There are various commercial metal cleaners specifically formulated to clean metal surfaces without causing corrosion or damage. These products are formulated for specific metals like stainless steel, aluminum, copper, and brass, ensuring effective cleaning while preserving the metal. Follow the manufacturer’s instructions for the specific cleaner. Typically, apply the cleaner to the metal surface, let it sit as recommended, and wipe clean.
Oxygen whitener, containing sodium percarbonate, is a natural alternative to bleach that is less toxic and safer for metal surfaces. Follow the package instructions to mix oxygen whitener with water. Apply the solution to the metal surface, scrub gently, and rinse.
Regularly inspecting and cleaning metal surfaces helps prevent damage and extends their lifespan. Conduct thorough inspections periodically to identify any signs of corrosion, discoloration, or wear.
Check metal surfaces for visible changes like rust spots, pitting, or tarnishing. Early detection allows for prompt corrective measures, minimizing the risk of extensive damage.
Use mild detergents or specialized metal cleaners to clean metal surfaces. Avoid abrasive materials that can scratch or dull the finish. Soft cloths or sponges are ideal for cleaning without causing damage.
Protective coatings effectively safeguard metal surfaces from environmental factors and corrosive cleaning agents.
Regular application of wax or polish can create a barrier that protects the metal from moisture and chemicals. This helps in maintaining the appearance and integrity of the surface.
For more durable protection, consider using clear lacquers or sealants. These coatings provide a robust shield against corrosive substances, including bleach, and can significantly extend the life of the metal.
Use appropriate cleaning agents and methods to avoid damaging metal surfaces.
If bleach is necessary, dilute it with water to reduce its potency. A common dilution ratio is ⅓ cup of bleach per gallon of water. This minimizes the risk of corrosion and pitting.
Limit the exposure time of bleach on metal surfaces. Apply the diluted bleach briefly, then rinse thoroughly with water to remove any residue. This prevents prolonged chemical reactions that can harm the metal.
Adopt preventive measures to maintain metal surfaces and protect them from damage caused by cleaning agents and environmental factors.
Ensure metal surfaces are dried thoroughly after cleaning. Moisture can lead to rust and corrosion, especially in metals like iron and steel. Regular drying helps maintain the metal’s integrity.
Maintain good ventilation in areas where bleach or other cleaning agents are used. Proper airflow helps disperse fumes that can interact with metal surfaces and cause corrosion.
Consider using alternative cleaning agents that are less corrosive than bleach.
A mixture of vinegar and baking soda can effectively clean metal surfaces without causing damage. Apply the paste to the surface, scrub gently, and rinse thoroughly.
Use commercial cleaners specifically designed for metals. These products are formulated to clean without causing corrosion or discoloration.
Implement best practices to ensure the longevity and appearance of metal surfaces.
Before applying any cleaning agent, perform a spot test on a small, inconspicuous area. This helps determine if the cleaner will cause any adverse reactions.
When using bleach or other harsh chemicals, cover metal fixtures to protect them from accidental exposure. This simple step can prevent significant damage.
Below are answers to some frequently asked questions:
When bleach, which primarily contains sodium hypochlorite (NaOCl), interacts with metal surfaces, it induces oxidation reactions. This occurs because sodium hypochlorite is a strong oxidizing agent. When bleach comes into contact with metals like iron, aluminum, copper, and brass, it causes these metals to lose electrons and form metal oxides. For example, iron reacts with sodium hypochlorite to form iron oxide, or rust, particularly in the presence of moisture.
This oxidation process weakens the metal structure, leading to corrosion, rust, and degradation. Even metals with protective layers, like stainless steel, can suffer damage from prolonged bleach exposure, resulting in pitting corrosion. The reaction with aluminum is rapid and severe, causing significant surface damage and pitting. Copper and brass tarnish and degrade due to bleach’s oxidizing properties. Therefore, it’s essential to understand and mitigate the effects of bleach on metals to preserve their integrity and longevity.
Bleach, primarily composed of sodium hypochlorite, significantly affects various metals due to its strong oxidizing properties. The metals most susceptible to bleach include:
To prevent damage, it is crucial to use diluted bleach solutions, limit exposure time, rinse thoroughly after use, and apply protective coatings.
Recognizing signs of corrosion on metal surfaces is vital for preventing extensive damage. Corrosion, the deterioration of metal due to chemical reactions with the environment, can manifest in several ways. Look for rust formation, which appears as reddish-brown flakes on iron and steel. Discoloration is another indicator, with metals like aluminum, copper, and brass turning dull or forming a green patina. Powdery deposits, such as white or green stains on copper and bronze, also signal corrosion. Additionally, pitting and cracking can occur, weakening the metal. Lastly, corroded metal may become brittle and prone to breaking. Being aware of these signs helps in early detection and mitigation of corrosion on metal surfaces.
To prevent bleach damage on metal surfaces, there are several protective measures you can take. First, always use diluted bleach solutions to minimize its corrosive effects. Typically, a mixture of ⅓ cup of bleach with 1 gallon of water is recommended. Limit the exposure time by ensuring that bleach does not remain on the metal surface for more than a few minutes. After cleaning, thoroughly rinse the metal surfaces with warm water to remove any bleach residue. Applying protective coatings such as wax, oil, or specialized metal protectants can create a barrier against bleach’s corrosive properties. Additionally, consider using alternative cleaning agents like isopropyl alcohol, soap and water, baking soda paste, or vinegar, which are less harsh on metals.
Bleach can cause corrosion, rust, and discoloration on metal surfaces due to its strong oxidizing properties. Therefore, safer alternatives for cleaning metal surfaces include:
These alternatives provide effective cleaning solutions while preserving the integrity of metal surfaces.
To maintain and care for metal surfaces to prevent damage, particularly from bleach, it is essential to follow several key strategies. First, apply protective coatings or finishes to create a barrier against corrosive substances. Regularly inspect metal surfaces for early signs of corrosion or damage, addressing any issues promptly to prevent moisture entry. Ensuring adequate lubrication can also help prevent metal-on-metal friction and subsequent oxidation.
When using bleach, always dilute it with water to lessen its corrosive effects and limit the exposure time. After using bleach, thoroughly rinse the metal surfaces with water to remove any residual chemicals. Testing bleach on a small, inconspicuous area before broader application can help avoid unexpected damage.
Routine maintenance should include regular cleaning and the reapplication of protective coatings as needed. By implementing these preventive measures, you can extend the lifespan of metal surfaces and reduce the risk of damage from bleach and other corrosive substances.

