Bleach is a household staple, praised for its powerful disinfecting properties, but did you know it can wreak havoc on certain metals? From tarnishing shiny surfaces to triggering corrosive damage, bleach’s effects on metal are far from harmless. Whether you’re cleaning stainless steel appliances, aluminum cookware, or copper fixtures, understanding how bleach interacts with these materials is crucial to avoid costly mistakes and preserve their integrity.
But what exactly makes bleach so damaging to metal? And are there safer alternatives for achieving the same sparkling results? In this article, we’ll explore how bleach impacts different types of metals, why corrosion occurs, and practical ways to protect your belongings. Could a simple change in your cleaning routine save your metals from unnecessary wear and tear? Let’s find out.
Bleach is a widely used cleaning agent known for its disinfecting power. It is primarily made up of sodium hypochlorite, a chemical renowned for its strong disinfecting properties. While bleach is effective at killing germs and removing stains, its interaction with metal surfaces can lead to significant damage.
Bleach reacts with metal through oxidation. In this chemical reaction, metal atoms lose electrons and form metal oxides. This process is crucial to understanding how bleach affects different types of metal surfaces.
For metals like iron and steel, oxidation accelerates rust formation, especially in moist environments, weakening the metal’s structure. Rust is particularly problematic in environments where moisture is present, as it can rapidly degrade the integrity of the metal.
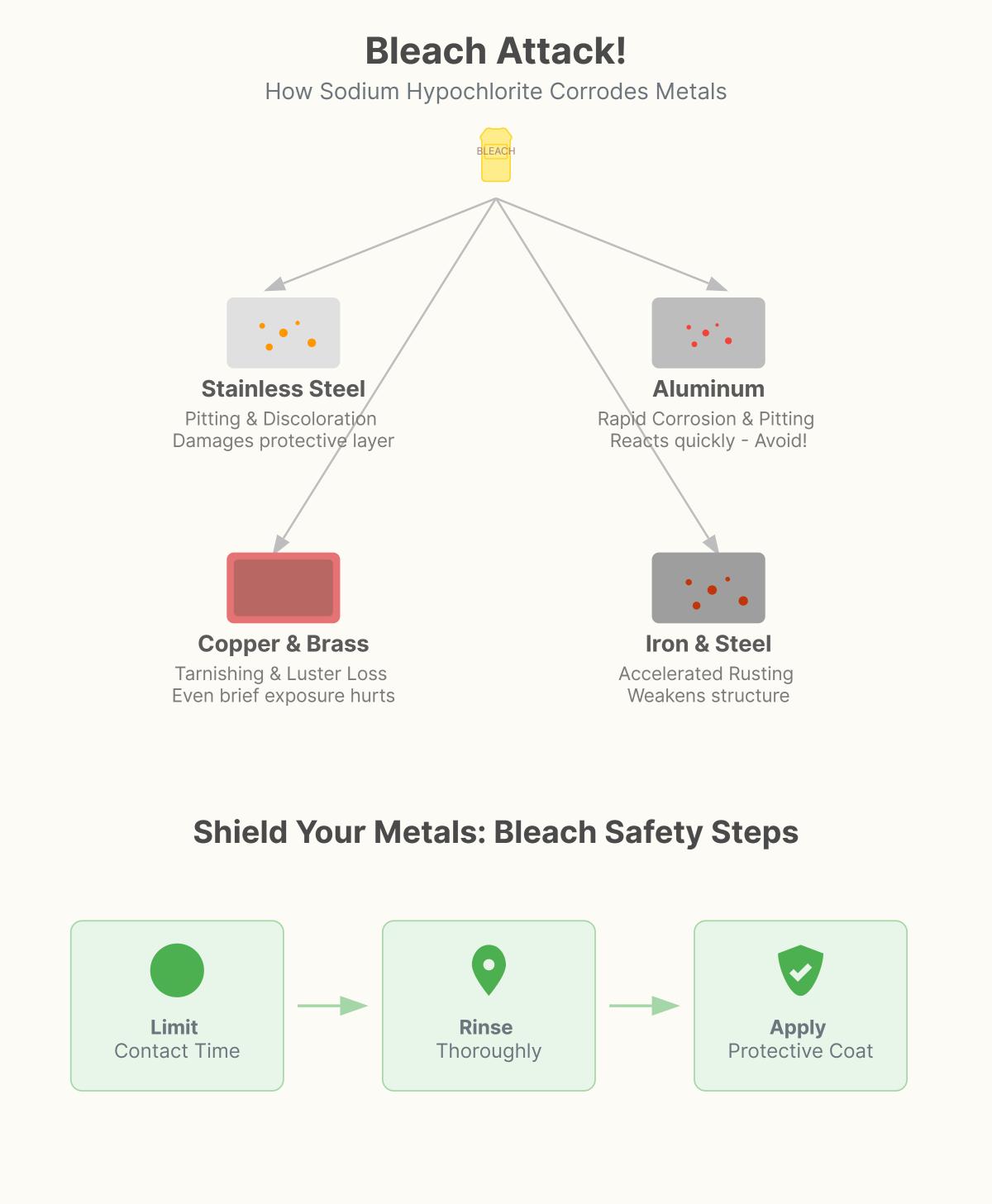
Stainless steel, known for its resistance to rust and corrosion, is not immune to the effects of bleach. The protective layer of chromium oxide that shields stainless steel can be damaged by bleach, leading to pitting corrosion and discoloration. This is especially true for commonly used grades like 304L and 316.
Aluminum is highly susceptible to corrosion when exposed to bleach. The interaction can cause pitting, which is the formation of small holes or pits on the metal surface. This not only affects the appearance of aluminum but also its structural strength.
Copper and brass, valued for their appearance and conductivity, can tarnish and lose their shine when exposed to bleach. The chemical reaction results in the formation of oxides on the surface, which can compromise the metal’s look and longevity.
To minimize the damage bleach can cause to metal surfaces, several preventive measures can be taken:
By understanding these basic principles and taking appropriate precautions, the detrimental impact of bleach on metal surfaces can be significantly mitigated.
Bleach, a common disinfectant and stain remover, is mainly made of sodium hypochlorite (NaOCl). Sodium hypochlorite is a strong oxidizing agent, giving it the ability to effectively eliminate bacteria, viruses, and other pathogens. This makes it an essential cleaning product in households and various industries.
When dissolved in water, sodium hypochlorite releases chlorine, which interacts with microorganisms to destroy their cell walls and internal structures, resulting in their death. This powerful chemical reaction is what gives bleach its remarkable disinfecting properties.
Bleach is available in various concentrations, with household products typically containing 3% to 6% sodium hypochlorite, while industrial formulations may go up to 12%. The concentration determines its strength and suitability for different cleaning tasks, including its potential impact on surfaces.
In addition to sodium hypochlorite, bleach formulations often include other components to enhance performance and stability:
The stability of sodium hypochlorite in bleach is affected by several factors:
The oxidizing nature of sodium hypochlorite makes it highly effective for disinfection but also poses a risk to metals. When bleach contacts metals, it can cause oxidation, leading to corrosion and deterioration of materials such as iron, aluminum, and copper. This characteristic highlights the importance of using bleach cautiously on metal surfaces to avoid damage.
Exposure to bleach causes pitting corrosion in stainless steel, creating small holes that damage its appearance and weaken its structure. Common grades like 304L and 316 are susceptible, with 316 being slightly more resistant due to its higher molybdenum content.
Aluminum is highly susceptible to bleach. When they come into contact, a chemical reaction occurs, causing corrosion and pitting. It is generally recommended to avoid using bleach on aluminum surfaces to prevent this kind of damage.
Copper and its alloy brass also react poorly to bleach. The strong oxidizing properties of bleach cause tarnishing and discoloration, degrading their appearance and reducing their longevity.
Iron and steel rust quickly when exposed to bleach, which accelerates the oxidation process. This rapid rust formation not only tarnishes their appearance but also weakens their structure significantly. In environments where these metals are exposed to moisture, the use of bleach can cause severe and rapid corrosion.
Galvanized steel is protected by a zinc coating, but bleach reacts with zinc to form zinc oxide. This reaction dulls the surface and reduces the coating’s protective qualities. Over time, this can lead to the underlying steel becoming exposed and prone to rust.
To minimize the damaging effects of bleach on various metals, consider the following strategies:
By understanding the specific effects of bleach on different metals and implementing these preventative measures, you can protect metal surfaces from the corrosive impact of bleach.
Corrosion occurs when metals break down over time due to chemical reactions with their surroundings. This typically involves the oxidation of metals, leading to the formation of oxides or other compounds. Corrosion weakens metal structures, reduces their functionality, and can lead to significant safety hazards. Understanding and preventing corrosion is crucial for maintaining the integrity and longevity of metal surfaces, especially when they are exposed to harsh chemicals like bleach.
Bleach, made of sodium hypochlorite, is a strong oxidizer that speeds up oxidation when it touches metal surfaces. This leads to damage like pits, discoloration, and weakened metal. For example, stainless steel can suffer from pitting corrosion, while aluminum and copper can tarnish or develop pits.
Following safety standards is key to preventing corrosion and safely using bleach on metals. Organizations such as OSHA provide guidelines for handling and using chemicals like bleach in industrial settings. These standards help in:
By following these tips and adhering to safety standards, you can effectively prevent corrosion caused by bleach and maintain the integrity of metal surfaces.
Vinegar breaks down grime and tarnish, while baking soda gently scrubs away residues without scratching the metal. To use this natural solution, mix equal parts vinegar and water in a spray bottle, sprinkle baking soda on the metal surface, and spray the vinegar mixture over it. Gently scrub with a soft cloth or sponge, rinse thoroughly, and dry the surface to prevent water spots.
Lemon juice is a natural, effective cleaner that removes stains and polishes metal surfaces, especially copper and brass, restoring their shine and removing tarnish. Simply apply fresh lemon juice to the surface, let it sit for a few minutes, and scrub gently with a soft cloth or sponge. Rinse with water and dry completely to preserve the finish.
Hydrogen peroxide offers a safer disinfectant alternative to bleach, effectively cleaning metal surfaces without causing corrosion. Pour it directly onto the metal, let it sit for a few minutes, then wipe with a soft cloth or sponge. Finish by rinsing with water and drying thoroughly.
Many commercial metal cleaners are designed to gently remove dirt, stains, and tarnish without causing corrosion. Always follow the manufacturer’s instructions and use a soft cloth or sponge to ensure the metal remains undamaged.
For routine cleaning, use mild detergents or soap and water, and avoid harsh abrasives that can scratch metal. Always clean in the direction of the metal’s grain to prevent scratches. For delicate or heavily tarnished metal surfaces, professional cleaning services may be a worthwhile option.
While bleach is a strong cleaning agent, it can damage metal surfaces due to its chemical nature. Sodium hypochlorite, the active ingredient in bleach, is a potent oxidizer that can corrode, discolor, or weaken metals. Proper precautions are essential to minimize these risks and preserve the integrity of metal surfaces.
Always wear gloves, goggles, and suitable clothing when using bleach to prevent skin and eye irritation or chemical burns. Additionally, ensure the area is well-ventilated to reduce exposure to fumes that can irritate the respiratory system.
To reduce the corrosive effects of bleach, always mix bleach with water before using it. A common dilution ratio is ⅓ cup of bleach per gallon of water, which balances cleaning effectiveness while minimizing damage to metals. Limit the contact time between bleach and metal surfaces, as prolonged exposure increases the likelihood of corrosion. After using bleach, rinse the metal surface well with clean water to wash away any leftover chemicals.
Applying protective coatings, such as wax or anti-corrosion sprays, can provide an extra layer of defense against bleach’s damaging effects. Test a small, hidden area before using bleach on a large surface to ensure it won’t cause damage. For metals like stainless steel, cleaning along the grain can help maintain the finish and avoid scratches.
Gentler alternatives like vinegar, baking soda, or hydrogen peroxide may be better suited for cleaning metal surfaces, as they are less likely to cause corrosion while still delivering effective results. Regular maintenance and inspections can also help detect early signs of damage and prevent long-term issues.
Below are answers to some frequently asked questions:
Bleach, primarily composed of sodium hypochlorite, is a strong oxidizing agent that can significantly affect various metals. For stainless steel, bleach can damage its protective chromium oxide layer, leading to pitting corrosion and discoloration. Even diluted bleach can cause significant harm if left in contact for extended periods. Aluminum reacts quickly with bleach, causing rapid corrosion and pitting, thus it’s generally advised to avoid using bleach on aluminum surfaces. Copper and brass are also vulnerable, with bleach causing tarnishing and loss of luster even after brief exposure. For iron and steel, bleach accelerates the rusting process, weakening the metal structure, especially in humid environments. To minimize damage, it’s essential to limit bleach contact time, thoroughly rinse with water, and consider applying protective coatings to the metal surfaces.
There are several safe alternatives to bleach for cleaning metal surfaces that can avoid the harmful effects such as corrosion and discoloration. Baking soda is an excellent choice; it acts as a mild abrasive and can be mixed with water to form a paste for scrubbing metal surfaces. White vinegar, a natural acid, is effective for cleaning and disinfecting metals like brass and copper without the harshness of bleach. Hydrogen peroxide serves as a gentler disinfectant, particularly for stainless steel. Sodium percarbonate, found in oxygen-based cleaners, offers a powerful cleaning effect without the corrosive properties of traditional bleach. A diluted ammonia solution (one part ammonia to ten parts water) can clean metals like galvanized steel effectively. Additionally, commercial metal cleaners are specifically designed to avoid corrosion and are suitable for various types of metals. Using these alternatives ensures that metal surfaces remain clean and intact without the damaging effects associated with bleach.
Yes, bleach can damage stainless steel. Bleach, which contains sodium hypochlorite (NaOCl), is a powerful oxidizing agent and disinfectant. While stainless steel is known for its corrosion resistance due to a protective chromium oxide layer, bleach can compromise this layer. The chlorine in bleach reacts with the chromium in stainless steel, forming chromium chloride, which weakens the protective layer. This can lead to pitting corrosion, where small holes form on the surface of the stainless steel, potentially damaging its appearance and structural integrity. To prevent this, it is advisable to avoid prolonged contact between bleach and stainless steel and to rinse the metal thoroughly if bleach is used.
Industrial safety standards address the use of bleach on metals by emphasizing caution due to its corrosive nature. Bleach, primarily composed of sodium hypochlorite, is a strong oxidizer that can cause significant damage to various metals, leading to corrosion, rust, or discoloration.
To mitigate these risks, standards such as those from the Occupational Safety and Health Administration (OSHA) classify bleach as a hazardous substance under the Hazard Communication Standard. This requires proper labeling, handling, and safety measures. Key guidelines include diluting bleach to reduce its corrosive effects, ensuring adequate ventilation to avoid fume buildup, and using personal protective equipment (PPE) such as gloves and safety glasses to prevent direct contact with the skin and eyes.
Additionally, it is advised to perform spot tests on inconspicuous areas before widespread application, thoroughly rinse metal surfaces after bleach exposure, and apply protective coatings to shield the metal. Storing bleach in well-ventilated areas and disposing of it according to local regulations are also critical practices. These measures help industries maintain safe and effective cleaning processes while protecting metal surfaces from damage.
Sodium dichloroisocyanurate (SDIC) offers several benefits for cleaning, particularly when compared to bleach. Firstly, SDIC is highly effective against a wide range of microorganisms, including bacteria, viruses, and fungi, ensuring rapid and lasting disinfection. Its high chlorine content contributes to its efficiency, making it suitable for various applications such as swimming pools, drinking water treatment, and surface disinfection.
Moreover, SDIC is stable under normal storage conditions, providing a long shelf life and consistent performance. This stability, combined with its high chlorine content, makes SDIC a cost-effective option, as it delivers long-lasting disinfection at a relatively low cost.
Another advantage of SDIC is its ease of use; it dissolves quickly in water and does not require specialized equipment for application. Additionally, when used appropriately, SDIC breaks down into harmless by-products, making it environmentally friendly.
Importantly, SDIC does not cause corrosion to metals, unlike bleach, which can damage metals like stainless steel. This makes SDIC a safer choice for environments where metal equipment is used, as it does not pose a risk to surfaces.
To minimize the risk of corrosion when using bleach on metal, follow these key steps:
By adhering to these practices, you can effectively clean metal surfaces with bleach while minimizing the risk of corrosion.

