When it comes to achieving a flawless metal finish, two terms often come up: electropolishing and electroplating. But what exactly sets these two processes apart? Both methods play a crucial role in various industries, from aerospace to medical devices, yet they serve distinct purposes and offer unique benefits. Electropolishing, known for its ability to enhance surface smoothness and brightness, contrasts sharply with electroplating, which excels in adding protective layers to metals. If you’re wondering which technique provides superior corrosion resistance or which is more cost-effective, this article will delve into the intricacies of both processes. By the end, you’ll have a comprehensive understanding of how these methods differ and which one is best suited for your specific needs. Ready to uncover the secrets behind these essential metal finishing techniques? Let’s dive in.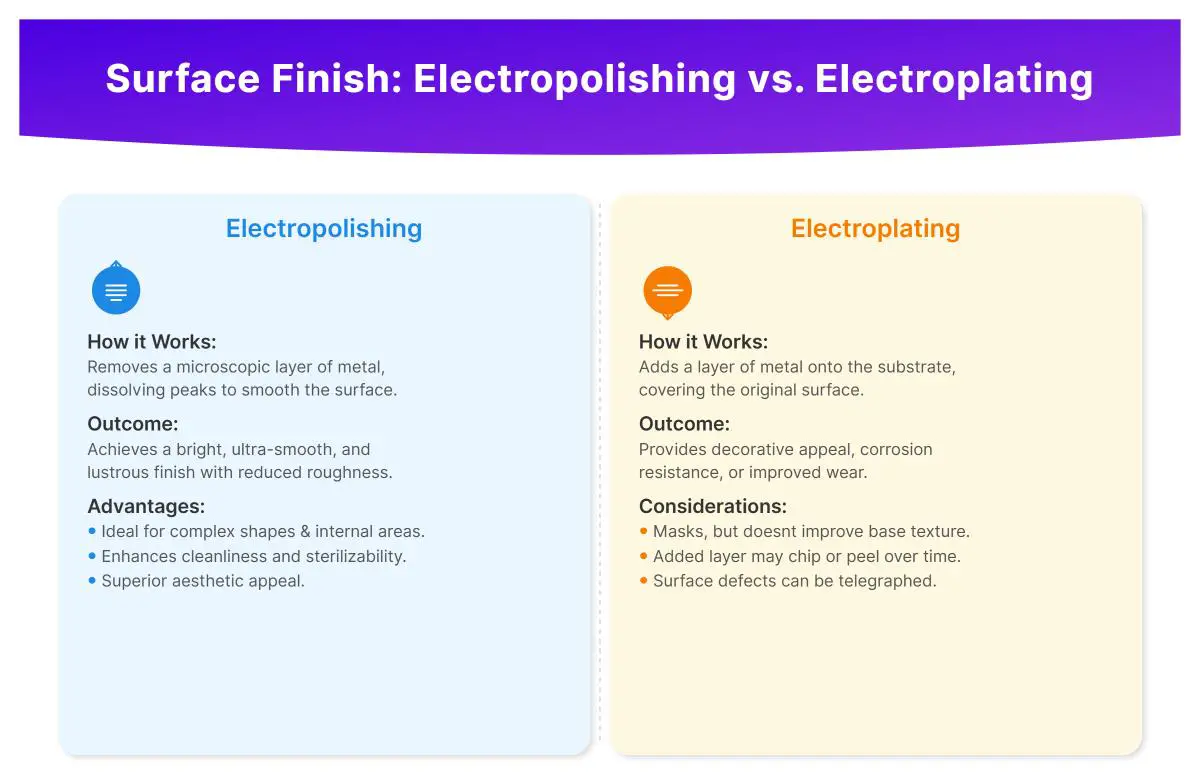
Metal surface finishing encompasses various processes that improve the appearance, durability, and performance of metal components. This finishing is critical in numerous industries, including aerospace, automotive, and medical equipment, where the quality of the metal surface can significantly impact the functionality and longevity of the products. These techniques ensure metal parts meet specific standards by improving surface properties.
Several techniques are employed in metal surface finishing, each with unique processes and outcomes. Two prominent methods are electropolishing and electroplating, which are often compared for their distinct advantages and applications.
Electropolishing is an electrochemical process that smooths and polishes the metal surface by removing a thin layer of material. The workpiece is made anodic, and as it loses ions, surface imperfections are leveled out. This process is particularly beneficial for enhancing the corrosion resistance and sanitary quality of metals, making it ideal for industries requiring high precision and cleanliness.
Key benefits of electropolishing include:
Electroplating, on the other hand, involves depositing a thin layer of metal onto the surface of a substrate. The workpiece is made cathodic, and metal ions from a solution are reduced and deposited onto the surface. This technique is often used to enhance the durability, wear resistance, and appearance of metal components.
Key benefits of electroplating include:
Understanding the differences between electropolishing and electroplating is essential for selecting the appropriate technique based on specific application needs.
Electropolishing tends to produce a smoother and more reflective surface compared to electroplating, which can sometimes result in a rougher finish depending on the plating material and process control.
Electropolishing excels in creating a bright, mirror-like finish, making it preferable for applications where aesthetic quality is a priority. Electroplating can also achieve a bright finish, but the smoothness largely depends on the substrate’s initial condition and the plating process parameters.
Electropolishing removes surface defects by leveling micro-peaks and valleys, resulting in a uniform surface. Electroplating, conversely, can mask surface defects by adding a layer of metal, but it may also highlight imperfections if the substrate is not adequately prepared.
Both techniques enhance corrosion resistance, but they do so differently. Electropolishing improves the metal’s inherent corrosion resistance by creating a passive oxide layer. Electroplating adds a protective layer that can prevent corrosive elements from reaching the substrate.
Electropolishing removes contaminants and creates a smooth, passive surface less prone to corrosion initiation. Electroplating provides a physical barrier that protects the underlying metal from corrosive environments.
Electropolished surfaces are less likely to deteriorate over time since they do not rely on an additional layer that can chip or peel. Electroplated coatings can provide excellent durability but may wear off or peel under certain conditions, necessitating maintenance or reapplication.
The cost of electropolishing and electroplating can vary based on the complexity of the process, the size of the components, and the materials involved. Generally, electropolishing may have higher upfront costs due to the need for precise control of the electrochemical process, while electroplating costs can fluctuate based on the type of metal being deposited.
Electropolishing is typically more energy-intensive due to the electrochemical reactions involved, but it can be more material-efficient as it removes only a thin layer. Electroplating consumes both energy and plating materials, and the efficiency can vary based on the bath composition and plating conditions.
Sustainability considerations are increasingly important in the selection of metal finishing techniques. Electropolishing tends to generate less hazardous waste compared to electroplating, which often involves the use of toxic metals and chemicals that require careful disposal and management.
Understanding these aspects of metal surface finishing helps make informed decisions for specific industrial needs and environmental considerations.
Electropolishing is an electrochemical technique that refines a metal surface, resulting in a smooth and polished finish. This process is primarily used for metals such as stainless steel, aluminum, and titanium. During electropolishing, the metal workpiece is immersed in an electrolyte solution and connected as the anode. A direct current (DC) is applied, causing the surface material to dissolve selectively, smoothing out micro-peaks and valleys.
Electropolishing operates through controlled anodic dissolution. First, the metal part is submerged in an acidic electrolyte solution, typically composed of sulfuric and phosphoric acids. When the DC current is applied, the metal atoms at the surface oxidize and dissolve into the electrolyte, which smooths out surface irregularities. This dissolution preferentially targets the peaks of surface asperities, leading to a leveling effect that enhances smoothness and reduces roughness. The result is a mirror-like, highly polished surface.
Electropolishing offers several significant benefits, making it a preferred choice for various high-precision industries:
Electropolishing is widely utilized across various industries due to its ability to produce ultra-smooth, clean, and corrosion-resistant surfaces. Key applications include:
While electropolishing removes material to enhance surface quality, electroplating involves adding a layer of metal onto the substrate. These processes differ significantly in their goals, mechanisms, and outcomes:
Both processes have their distinct advantages and are chosen based on specific application requirements and desired outcomes.
Electroplating is a technique where a thin layer of metal is deposited onto a material’s surface using an electric current. This process enhances the properties of the base material, providing improved durability, corrosion resistance, and aesthetic appeal.
The electroplating process involves several key steps:
Electroplating provides several significant advantages, including improved corrosion resistance and enhanced durability:
Electroplating is widely applied across various industries, each utilizing the process to meet specific requirements:
While electroplating adds a metal layer to the surface, electropolishing removes material to create a smooth finish. Here are the key differences:
Electropolishing is a process that uses electrolysis to remove metal from a surface, resulting in a smoother and brighter finish. It primarily improves surface quality, enhances corrosion resistance, and ensures cleanliness. This technique does not add any material; instead, it refines the existing surface by selectively dissolving high points to create a uniform finish. It is particularly useful in industries that require high precision and aesthetic appeal.
Conversely, electroplating involves depositing a thin layer of another metal onto a substrate through an electrochemical process. This process enhances durability, provides corrosion protection, and improves the appearance of metal parts. Unlike electropolishing, electroplating adds material, which can be different from the base metal, offering a wide range of properties depending on the coating used.
Electropolishing greatly improves surface quality by removing impurities and inclusions. This process results in a smooth, bright finish that is aesthetically superior and suitable for complex geometries. The elimination of micro-peaks and valleys leads to a highly reflective surface, making it ideal for applications where visual appeal is critical.
Electroplating can also provide a smooth and bright finish, but the quality largely depends on the initial condition of the substrate and the plating parameters. While it can hide some imperfections, the finish may not be as smooth or shiny as electropolishing. The added metal layer can sometimes introduce variability in surface roughness.
Electropolishing enhances the inherent corrosion resistance of metals, particularly stainless steel, by creating a passive oxide layer. This layer is free of surface impurities and inclusions that could act as corrosion initiation sites. Therefore, electropolished surfaces can be up to 30 times more resistant to corrosion than untreated ones.
Electroplating provides corrosion resistance by adding a protective metal layer that shields the underlying substrate from corrosive elements. The effectiveness of this protection depends on the type of metal used for plating and the quality of the plating process. However, the protective layer can potentially chip or peel over time, reducing its effectiveness.
The durability of electropolished surfaces is inherent to the base material, as no additional layers are added. This process results in a surface that is less prone to corrosion and easier to maintain. However, the smooth finish can be susceptible to visible scratches, which may affect its appearance but not its structural integrity.
The durability of electroplated surfaces depends on the thickness and quality of the plating layer. While electroplating can significantly enhance wear resistance and extend the lifespan of components, the added layer may wear off or peel under certain conditions, necessitating maintenance or reapplication.
The cost of electropolishing and electroplating varies based on the complexity of the process, the size of the components, and the materials involved. Electropolishing may have higher upfront costs due to the need for precise control of the electrochemical process, while electroplating costs can fluctuate based on the type of metal being deposited.
Electropolishing tends to be more energy-intensive due to the electrochemical reactions involved. However, it can be more material-efficient as it removes only a thin layer of material. Electroplating consumes both energy and plating materials, and its efficiency can vary based on the bath composition and plating conditions.
Electropolishing generally generates less hazardous waste compared to electroplating. The process primarily involves the use of acidic electrolyte solutions, which can be managed with proper waste disposal methods. It tends to be more environmentally friendly due to the minimal use of toxic metals.
Electroplating often involves the use of toxic metals and chemicals, which require careful handling and disposal to minimize environmental impact. The process can generate significant waste, including spent electrolyte solutions and metal sludge, which need to be treated to prevent environmental contamination.
Start by thoroughly cleaning the metal surface to remove oils, dirt, and oxides, ensuring uniform contact with the electrolyte solution and preventing contamination. After cleaning, rinse the workpiece to remove any residual cleaning agents and dry it completely. This step is crucial for ensuring the effectiveness of the electropolishing process.
Position the workpiece as the anode in the electrolytic cell, and select an electrolyte solution suitable for the metal being processed, such as a mix of phosphoric and sulfuric acids for stainless steel. Use an inert cathode material such as stainless steel or lead to complete the circuit.
After plating, rinse the workpiece thoroughly to remove any leftover electrolytes. Sometimes, additional treatments such as heat treatment or passivation are applied to enhance the properties of the plating layer. Inspect the workpiece for uniform coating and any defects that may have occurred during the plating process.
Always wear appropriate PPE, including gloves, goggles, and protective clothing, to safeguard against chemical exposure. Ensure proper ventilation in the workspace to avoid inhalation of fumes. Follow safety protocols for handling and disposing of chemicals used in electropolishing and electroplating. Use an electrolytic cell setup with a DC power supply, appropriate electrolyte solutions, agitation systems, and temperature control mechanisms to maintain optimal processing conditions.
Electropolishing and electroplating are extensively utilized in the aerospace industry to enhance the performance and longevity of critical components.
Electropolishing is especially beneficial for aircraft engine parts and structural elements that are exposed to harsh environments. This process produces ultra-smooth, corrosion-resistant surfaces, reducing friction and wear and thereby extending the lifespan of these components. Additionally, electropolishing helps reduce the risk of stress corrosion cracking, crucial for maintaining the structural integrity of aerospace parts.
Electroplating is commonly used to apply protective and functional coatings to aerospace components. For instance, chromium plating on landing gear and turbine blades enhances wear resistance and corrosion protection. Nickel and cadmium plating provide additional corrosion resistance and improve the durability of parts subjected to extreme conditions. The ability to customize the coating material allows for tailored solutions to specific aerospace requirements.
In the automotive industry, both electropolishing and electroplating are employed to improve the performance and aesthetics of vehicle components.
Electropolishing is used to enhance the appearance and corrosion resistance of stainless steel exhaust systems, trim, and other visible components. The process produces a bright, reflective finish that is not only visually appealing but also easier to clean and maintain. Electropolishing also smooths engine components, which improves fuel efficiency and reduces emissions by minimizing friction.
Electroplating is widely used in the automotive sector to protect parts from corrosion and wear. Zinc plating is applied to steel components to prevent rusting, while chrome plating is used for decorative finishes on bumpers, wheels, and trim. Nickel plating is often utilized for under-the-hood components to enhance their durability and resistance to harsh operating conditions. These coatings extend the lifespan of automotive parts and contribute to their aesthetic appeal.
The medical industry relies heavily on both electropolishing and electroplating to ensure the safety, durability, and cleanliness of medical devices and instruments.
Electropolishing is crucial for producing biocompatible and sterile surfaces on medical instruments, implants, and devices. The process removes surface contaminants and creates a smooth, passive oxide layer that resists bacterial adhesion and is easy to sterilize. Surgical instruments, orthopedic implants, and stents are often electropolished to meet stringent hygiene standards and ensure patient safety.
Electroplating is used to deposit biocompatible metals, such as gold and platinum, onto medical devices to improve their functionality and durability. For example, electroplated coatings on pacemaker components enhance electrical conductivity and corrosion resistance. Dental implants and other prosthetics are also commonly electroplated to improve their appearance and biocompatibility, ensuring that they perform effectively within the human body.
Electropolishing and electroplating find applications in various other industries, each leveraging the unique benefits of these processes to meet specific requirements.
Below are answers to some frequently asked questions:
Electropolishing improves surface finish by removing a thin, uniform layer of metal from the workpiece, thereby smoothing out microscopic surface irregularities and reducing surface roughness. This process results in a bright, smooth, and lustrous finish, which is particularly effective on complex geometries, including recessed or internal areas. By selectively dissolving microscopic peaks, electropolishing enhances the cleanliness and aesthetic appeal of the metal surface, making it easier to sterilize and maintain.
In contrast, electroplating adds a metal layer onto the surface of the substrate. While this can provide decorative appeal, corrosion resistance, or improved wear properties, it does not inherently improve the base metal’s surface texture. Instead, electroplating masks underlying surface defects and may add a layer that can chip or peel over time.
Electropolishing generally provides better corrosion resistance compared to electroplating. This is primarily because electropolishing removes a uniform layer from the metal surface, eliminating micro-imperfections, contaminants, and inclusions that can initiate corrosion. This process also enriches the surface with a passive chromium oxide layer, particularly in stainless steel, which significantly enhances its corrosion resistance.
In contrast, electroplating involves depositing a foreign metal layer onto the substrate, which can offer temporary protection but is prone to cracking, chipping, or galvanic corrosion if the coating is compromised. While electroplating can be effective, its performance is highly dependent on the integrity of the coating, making it less reliable over the long term, especially in harsh environments.
Therefore, for applications requiring high durability and long-term exposure to corrosive conditions, electropolishing is the superior choice.
Electropolishing and electroplating serve distinct purposes and are applied in various industrial sectors to enhance the properties of metal components.
Electropolishing is an electrochemical process that removes a thin layer from a metal surface to achieve a smoother and brighter finish. It is widely used in medical and dental instruments to reduce bacterial retention and improve smoothness. In the food and beverage industry, it provides sanitary surfaces for equipment, reducing contamination risks. Aerospace applications benefit from reduced surface roughness and friction, while the automotive industry sees increased lifespan and reduced corrosion in parts. Industrial equipment, such as reactor vessels and storage tanks, also utilize electropolishing for smoother operations.
Electroplating involves depositing a thin layer of material, typically metal, onto another metal surface using an electric current. This process enhances corrosion resistance, wear resistance, and aesthetic appeal. It is commonly used in consumer goods to improve the durability and appearance of household items and electronics. In aerospace and automotive sectors, electroplating provides corrosion protection and enhances mechanical properties of parts. It is also used in medical devices to add protective layers and in industrial machinery to improve wear resistance.
Electropolishing and electroplating both have distinct environmental impacts due to their processes and by-products. Electropolishing generates wastewater containing dissolved metal ions like iron, chromium, nickel, and copper. This wastewater is toxic to aquatic life but can be effectively treated through neutralization and sorption, significantly reducing its environmental impact. Additionally, electropolishing produces less abrasive waste compared to mechanical polishing, requiring fewer cleaning agents and less energy.
Electroplating, on the other hand, produces a higher volume of wastewater with high concentrations of hazardous substances from the plating baths and rinsing processes. This wastewater contains heavy metals that are highly toxic and require complex treatment processes to prevent environmental contamination. The treatment typically involves precipitation, filtration, and chemical detoxification.
Choosing between electropolishing and electroplating for your project involves assessing the specific requirements and desired outcomes for your metal surfaces.
Electropolishing is ideal when you need a smooth, bright, and corrosion-resistant finish without altering the metal’s original properties. It is particularly effective for complex geometries and internal passages, making it suitable for industries like pharmaceuticals, food processing, and medical devices where hygiene and cleanliness are paramount. Electropolishing enhances the surface by removing micro-roughness and impurities, thus improving corrosion resistance and making the surface easier to clean.
On the other hand, electroplating is the process to choose if you need to apply a specific metal coating to enhance surface properties such as wear resistance, electrical conductivity, or for decorative purposes. This method is suitable when the application demands a change in surface composition, and the added coating thickness is acceptable. Electroplating is versatile in terms of the variety of metals and finishes it can apply, making it useful for automotive, electronics, jewelry, and aerospace industries.
When implementing electropolishing and electroplating processes, several critical safety measures must be taken to ensure the safety of personnel and equipment.
For both processes, workers should wear appropriate protective clothing, such as gloves, goggles, and masks, to guard against exposure to harmful chemicals and electrical hazards. Electrical safety is paramount; equipment must be kept dry and regularly inspected to prevent short circuits and ensure proper insulation of electrodes. Adequate ventilation is essential to remove hazardous fumes and prevent inhalation risks.
Electropolishing involves handling corrosive solutions, typically containing phosphoric and sulfuric acids. These solutions require careful storage and handling to prevent accidental spills and contact with electrical equipment. Regular maintenance of the electropolishing bath is necessary to avoid overheating and the formation of metal sludge, which can interfere with the process.
Electroplating poses risks related to fire hazards due to overheating and electrical malfunctions, as well as the release of hydrogen, which is highly flammable. Proper ventilation systems and non-combustible materials for equipment are crucial. Regular inspections of electrodes and connections help prevent corrosion and electrical failures.

