Imagine transforming a dull, tarnished piece of copper into a gleaming, mirror-like masterpiece with minimal effort. This is the magic of electropolishing—a technique that not only enhances the aesthetic appeal of copper but also improves its corrosion resistance and We’ll cover everything you need to know, including the optimal electrolyte composition, the necessary equipment, and the precise process parameters to ensure success. Whether you’re curious about the advantages of electropolishing over mechanical methods or looking for troubleshooting tips, this comprehensive guide has you covered. Ready to dive in and unlock the secrets to a flawless copper finish? Let’s get started.
Electropolishing is an advanced electrochemical process used to enhance the surface finish of metals. By selectively removing a thin layer from the metal’s surface, electropolishing achieves a smooth, clean, and corrosion-resistant finish. This process is particularly valuable in industries requiring high precision and pristine surface quality, such as electronics, aerospace, and medical devices.
Electropolishing involves several key steps and components to achieve the desired results:
The metal piece to be polished serves as the anode (positively charged), while the cathode (negatively charged) is usually made of stainless steel or lead. Both are immersed in an electrolyte solution, which is a specially formulated liquid that facilitates the electrochemical reaction.
When an electric current is applied, metal ions are dissolved from the anode’s surface into the electrolyte solution. This controlled removal smooths the microscopic peaks and valleys, creating a uniform, polished finish.
Electropolishing offers several benefits over traditional mechanical methods, including a smoother surface, enhanced corrosion resistance, precise material removal, and improved hygiene.
Electropolishing is used in various industries to achieve high-quality finishes. In electronics, it ensures reliable, clean surfaces. In aerospace, it enhances part durability. In medical devices, it provides smooth, sterile surfaces for implants and instruments. It also creates mirror-like finishes for decorative items.
Since Dr. Pierre Jacquet’s industrialization of electropolishing in the 1930s, advances in electrolyte formulations and process control have improved its efficiency and effectiveness, making it a preferred method for high-quality metal finishes in modern manufacturing.
Electropolishing is an advanced electrochemical process that improves the surface finish of metal parts by removing a thin layer of material.
In the electropolishing process, the metal part to be polished acts as the anode (positively charged) and is immersed in an electrolyte bath. The cathode (negatively charged) is typically made from materials like stainless steel or lead. The electrolyte solution facilitates the electrochemical reaction necessary for polishing.
An electric current is applied to the electrolyte bath, causing metal ions to dissolve from the surface of the anode into the solution. This controlled removal of material smooths out surface imperfections, resulting in a bright and polished finish.
One of the primary benefits of electropolishing is its ability to improve corrosion resistance. By removing surface imperfections where moisture can accumulate, it helps prevent corrosion and extends the lifespan of metal components. Additionally, electropolishing provides a smooth, reflective surface that enhances the aesthetic appeal of metal parts, resulting in a bright, mirror-like finish.
The electropolishing process effectively removes rust, embedded debris, and other contaminants without affecting the metal’s surface hardness, making it crucial for applications requiring high cleanliness, such as medical devices and food processing equipment.
Electropolishing significantly reduces microfinish values, resulting in a smoother surface, which is particularly important for components like valves and gears that benefit from improved performance and reduced wear.
Electropolishing is widely used across various industries to produce high-quality finishes, with common applications including medical devices, aerospace components, food processing equipment, and decorative items.
When it comes to copper, electropolishing is an effective method for achieving a flawless finish. It not only enhances the appearance of copper but also improves its durability and corrosion resistance, making it suitable for both functional and decorative applications.
Thoroughly cleaning and degreasing copper is essential before electropolishing. Any contaminants on the surface can affect the quality of the electropolished finish. Here are the detailed steps:
Surface pre-treatment ensures that the copper is in optimal condition for electropolishing. This involves inspection and careful handling of the parts.
Understanding the electropolishing process is essential for achieving the desired results. This includes the preparation of the electrolyte solution and controlling process parameters.
Several factors influence the success of the electropolishing process, including electrolyte composition, temperature control, and post-treatment.
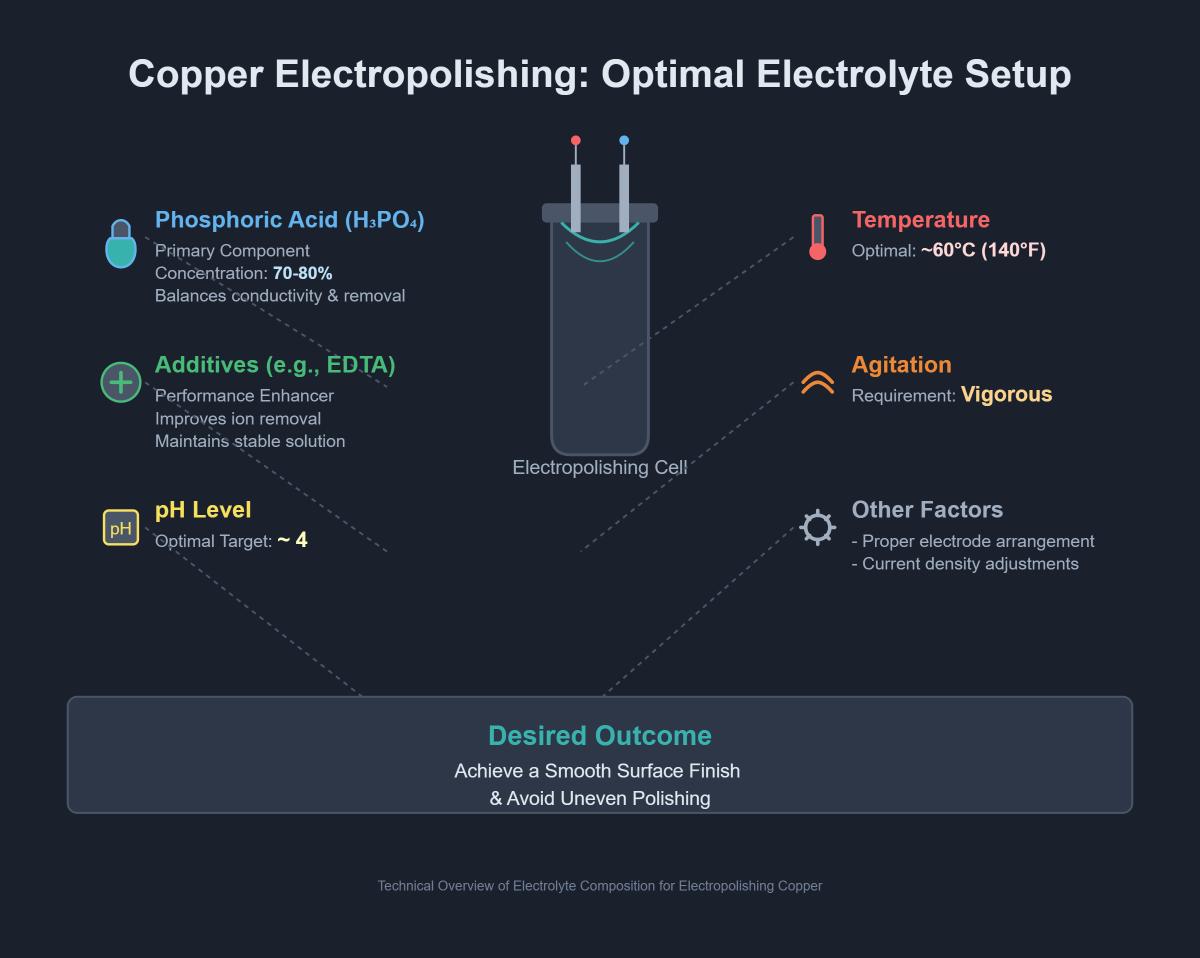
To achieve an optimal surface finish, electropolishing copper requires a precise combination of chemicals. The primary component of the electrolyte solution used in electropolishing copper is phosphoric acid, which is responsible for dissolving the copper ions and smoothing the surface.
Phosphoric acid is the main ingredient in the electrolyte solution for copper electropolishing. The concentration of phosphoric acid typically ranges from 60% to 90%, with the most effective results often found between 70% and 80%. This high concentration ensures efficient removal of the copper surface layer, resulting in a smooth and reflective finish.
To enhance the performance of the phosphoric acid solution, several additives can be included:
Working with the chemicals required for electropolishing copper involves several safety considerations. Proper handling and safety measures are essential to prevent accidents and ensure a safe working environment.
When handling chemicals like phosphoric acid and other additives, it is crucial to wear appropriate PPE:
Proper ventilation or fume extraction systems are essential to maintain air quality and protect workers’ health from the harmful fumes released by phosphoric acid and other chemicals.
Copper parts must be thoroughly cleaned before electropolishing to remove any contaminants that could interfere with the process. This involves methods such as vapor degreasing, alkaline cleaning, or acid pickling to ensure a clean surface.
Maintaining the electrolyte solution at an optimal temperature is critical for effective electropolishing. Typically, the temperature should be around 60°C (140°F). Consistent temperature control helps achieve uniform polishing and prevents the formation of unwanted oxide layers.
Adjusting the current density is essential to ensure even polishing across the entire surface of the copper part. The appropriate current density depends on the size and geometry of the part. Too high a current density can lead to over-polarization, while too low a current density may result in insufficient polishing.
Vigorous agitation of the electrolyte solution is necessary to maintain a consistent composition and prevent localized depletion of the electrolyte. Agitation helps in removing hydrogen bubbles that form during the process, ensuring a smooth and uniform finish.
During electropolishing, copper ions are oxidized at the anode and dissolve into the electrolyte solution. The primary anodic reaction involves the transfer of copper ions from the copper surface into the solution. Hydrogen gas is simultaneously evolved at the cathode, facilitating the reduction reaction.
While oxide layers can form during electropolishing, they should be minimized to achieve an optimal finish. Proper control of the electrolyte composition, temperature, and current density helps in reducing the formation of these layers.
After the electropolishing process, it is crucial to thoroughly rinse the copper parts with deionized water to remove any residual electrolyte. This step prevents any further chemical reactions that could degrade the surface finish.
Depending on the application, additional treatments like nitric or citric acid dips may be needed to remove any remaining films or residues. These treatments ensure that the final surface is clean and ready for use.
Electropolishing copper requires specific equipment to ensure efficiency and achieve the desired surface finish.
Use a non-reactive material like low-density polyethylene (LDPE) plastic for the electrolyte tank, as it resists the acids involved in the process.
A phosphoric acid-based solution is commonly used for electropolishing copper. The concentration of the phosphoric acid typically ranges from 60% to 90%, with an optimal range of 70% to 80%. This balance helps achieve good conductivity and effective material removal.
A direct current (DC) rectifier is essential to provide the necessary voltage and current for the electropolishing process. The operating voltage usually ranges from 6 to 20 volts, depending on the size and geometry of the copper parts being polished.
Use lead or stainless steel cathodes, and place them 2 to 6 inches from the workpiece to prevent etching and ensure uniform polishing.
To maintain a consistent temperature and electrolyte composition, an agitation system is required. This can be achieved using circulation pumps or air agitation, which help prevent localized depletion of the electrolyte and ensure uniform polishing.
Maintaining the optimal temperature of the electrolyte solution is crucial. Typically, steam coils or immersion heaters are used to keep the temperature around 60°C (140°F). Proper temperature control ensures effective polishing and prevents unwanted side reactions.
Setting up the electropolishing system involves several considerations to ensure the process runs smoothly and efficiently.
Before placing the copper workpiece in the electrolyte tank, thoroughly clean it using methods like vapor degreasing or alkaline cleaning to remove contaminants such as oils and oxides. For surfaces with significant oxidation, an acid pickling step may be necessary.
The workpiece should be securely positioned on a rack made from materials like copper, titanium, or plastisol-coated copper with titanium tips. Ensure the workpiece is at least 6 inches above the tank bottom to avoid contact with sludge and to allow for proper circulation of the electrolyte.
Maintain a distance of 2 to 6 inches between the cathode and the anode (workpiece) to prevent etching or pitting. This distance is critical for achieving a uniform electric field and consistent polishing results.
Proper ventilation is essential to remove the corrosive fumes generated during the electropolishing process, primarily hydrogen gas. Ensure the setup includes adequate ventilation or fume extraction systems to maintain a safe working environment.
The electrolyte solution should be maintained at approximately 60°C (140°F). Consistent temperature control is necessary for achieving optimal results and preventing the formation of oxides or other unwanted layers on the copper surface.
Adjust the current density based on the size and geometry of the copper parts. Proper current density ensures uniform polishing without over-polarization, which can lead to defects on the surface.
The electropolishing process can take a few minutes to 20 minutes, depending on the desired finish. Closely monitor the process to achieve the best surface quality.
After electropolishing, thoroughly rinse the copper parts with deionized water to remove any residual electrolyte. This step is crucial to prevent further chemical reactions that could affect the surface finish.
If necessary, neutralize any acidic residue on the copper parts with a mild alkaline solution. This helps stabilize the surface and prepare it for subsequent use or further processing.
Dry the copper parts completely using air blowers or lint-free cloths. This step is important to prevent water spots and ensure a pristine surface finish.
Always wear appropriate protective gear, including eye protection, chemical-resistant gloves, and a lab coat, to prevent exposure to corrosive substances.
Ensure proper ventilation to remove harmful gases, mainly hydrogen, produced during electropolishing. This is crucial for a safe working environment.
Handle acids with care. Always add acid slowly to water to avoid violent reactions. It is essential to have an acid spill kit available in case of accidental spills.
Proper surface preparation is crucial for effective electropolishing. The copper surface must be thoroughly cleaned of contaminants like grease, oils, and oxides. Cleaning methods include vapor degreasing (using solvents), alkaline soak cleaning (immersing in an alkaline solution), alkaline electrocleaning (using electrolytic processes), and mechanical or chemical deburring (to smooth rough edges).
The electrolyte solution, primarily phosphoric acid (H3PO4) with a concentration of 60% to 90%, should have a pH around 4. Additives like EDTA and alcohols (methanol, ethanol, or butanol) can improve the polishing quality.
Maintain an optimal temperature range of 80°F to 180°F (27°C to 82°C), ideally around 140°F (60°C). The recommended current density is 2.0 to 4.0 A/dm² (18.6 to 37.2 A/ft²) to ensure uniform material removal. Adjust voltage to maintain this current density, typically around 500 mV compared to a Saturated Calomel Electrode (SCE). Vigorous agitation is essential to keep the electrolyte composition consistent and prevent oxide layer formation.
In this process, the copper workpiece serves as the anode, while the cathode is usually made of stainless steel or zirconium. Optimize the distance between them to ensure uniform polishing and prevent defects.
Thoroughly rinse the copper parts to remove any residual electrolyte and dry them completely to prevent staining and corrosion, ensuring the polished surface maintains its integrity and appearance.
Several factors influence the efficiency and quality of the electropolishing process:
Thoroughly cleaning and preparing the copper pieces before electropolishing is crucial for achieving the best results.
Use vapor degreasing to remove oils and greases from the copper surface. Then, immerse the copper parts in an alkaline cleaning solution to eliminate remaining residues. Utilize an alkaline electrocleaning bath with an electric current for further cleaning, followed by an acid dip in nitric or citric acid if necessary to remove discoloration or oxidation. Rinse thoroughly with water.
Mix phosphoric acid with water to create an electrolyte solution with a pH of around 4. You can add EDTA for better results. Maintain the electrolyte bath temperature at approximately 60°C (140°F) to ensure efficient electropolishing. Connect the copper piece to the positive terminal (anode) of a DC power supply. The cathode (negative terminal) should be made of a suitable material such as stainless steel or lead. Keep the bath well-agitated to maintain a uniform electrolyte mixture and prevent oxide layer formation on the copper.
Place the cleaned copper piece into the electrolyte solution. Apply an electric current to initiate the electropolishing process. The current causes metal ions to dissolve from the anode (copper piece) into the electrolyte solution. Continuously monitor the temperature, electrolyte composition, and current density. Adjust parameters as needed to prevent over-polarization and ensure uniform polishing. Periodically inspect the copper part during the process to check for even polishing and address any issues that arise.
Rinse the polished copper piece thoroughly with deionized water to remove leftover electrolyte. If necessary, dip the copper part in a mild alkaline solution to neutralize any acidic residues. Dry the copper piece completely using air blowers or lint-free cloths to prevent water spots or recontamination. For a final clean finish, apply an acid dip (e.g., nitric or citric acid) to remove any remaining residues such as phosphates and sulfates.
Achieving the desired surface finish in copper electropolishing requires careful consideration of several critical factors. These factors ensure that the copper surface is smooth, shiny, and free of imperfections.
The electrolyte solution’s composition significantly impacts the surface finish quality. Typically, a phosphoric acid-based solution is used due to its effectiveness in dissolving copper ions and creating a smooth surface. The addition of specific additives such as butanol or glycerol can help control the viscosity of the solution, leading to a more uniform polish.
Maintaining an optimal current density, within the range of 9.3 to 37.2 A/ft², is crucial for a consistent surface finish. A lower current density may result in insufficient polishing, while a higher density can cause over-polarization, leading to surface defects. Adjust the current density according to the size and geometry of the copper part to ensure even material removal.
The temperature of the electrolyte bath should be kept around 60°C (140°F). Use a reliable heating system to avoid temperature fluctuations and ensure consistent polishing results. Consistent temperature control ensures that the electropolishing process proceeds smoothly, preventing the formation of unwanted oxide layers.
Agitating the electrolyte solution properly maintains a uniform composition and prevents localized depletion. Continuous agitation helps in removing hydrogen bubbles that form during the process, which can otherwise cause pitting on the copper surface. Using circulation pumps or air agitation systems can achieve the desired level of agitation.
To achieve a mirror-like finish on copper surfaces, follow these specific techniques:
Before electropolishing, mechanically polish the copper surface to remove any deep scratches or significant imperfections. This step helps in achieving a smoother base, which is essential for a high-quality electropolished finish.
Adjust the electrolyte composition, current density, and temperature precisely to create the optimal conditions for a mirror finish. Fine-tuning these parameters ensures that the surface is uniformly polished, with minimal defects.
After electropolishing, an acid dip using nitric or citric acid can remove any remaining residues and enhance the surface’s brightness. This final step ensures that the copper surface is clean and exhibits a high level of reflectivity.
During the electropolishing process, you may encounter various issues that can affect the surface finish. Here are some common problems and their solutions:
If the surface appears uneven, check the current density and agitation. Uneven current distribution or inadequate agitation can lead to localized polishing, causing an inconsistent finish. Adjust the current density and ensure proper agitation to achieve uniform polishing.
Pitting or etching can occur due to trapped hydrogen bubbles or impurities in the electrolyte. To prevent this, maintain continuous agitation and regularly filter the electrolyte to remove contaminants. Also, ensure that the copper surface is thoroughly cleaned before electropolishing.
Improper rinsing or leftover acidic residues can cause discoloration. After electropolishing, rinse the copper parts thoroughly with deionized water and, if necessary, neutralize any acidic residues with a mild alkaline solution. Proper drying techniques, such as using air blowers or lint-free cloths, can also help prevent discoloration.
By carefully managing these factors and techniques, you can achieve a high-quality, polished finish on copper parts through electropolishing.
Below are answers to some frequently asked questions:
The optimal electrolyte composition for electropolishing copper typically includes phosphoric acid as the primary component, often in concentrations between 70% and 80%. This concentration range provides an effective balance between conductivity and controlled material removal. Additives such as EDTA can be included to enhance performance by improving ion removal and maintaining a stable solution. The pH of the electrolyte should be adjusted to around 4 for optimal conditions. Additionally, maintaining the electrolyte temperature at approximately 60°C (140°F) and ensuring vigorous agitation are essential for achieving a smooth surface finish. Proper electrode arrangement and current density adjustments are also crucial to avoid uneven polishing.
To achieve a mirror finish using electropolishing, follow these essential steps:
Electropolishing offers several advantages over mechanical polishing, particularly for materials like copper. Firstly, electropolishing provides precision and uniformity by removing a consistent layer of surface material, ensuring a smooth and even finish across complex shapes where mechanical methods may struggle. This process also enhances corrosion resistance by eliminating surface impurities and improving the metal’s natural protective layer, which mechanical polishing may compromise by embedding contaminants. Additionally, electropolishing effectively deburrs and smooths edges, making it ideal for applications requiring high safety and precision, unlike mechanical polishing that may leave rough edges. It also results in an ultraclean surface, free from debris, which is crucial for industries like aerospace and pharmaceuticals. Lastly, electropolishing is more efficient and cost-effective for large-scale production, as it can process multiple parts simultaneously, whereas mechanical polishing is labor-intensive and less suited for mass production.
When handling chemicals for electropolishing copper, it is crucial to follow strict safety precautions to prevent accidents and ensure a safe working environment. Always wear appropriate personal protective equipment (PPE) such as impervious, chemical-resistant gloves, chemical safety goggles, a full-face shield, and a lab coat or apron. Conduct all operations in a well-ventilated area, preferably under a chemical hood, to avoid inhaling corrosive vapors and prevent the accumulation of hydrogen gas. Be prepared for emergency procedures: neutralize spills with lime or soda ash, and manage fires with suitable media like water spray or dry chemical. Maintain optimal temperatures and current density during the process to ensure efficiency and safety. After electropolishing, thoroughly rinse and dry the parts to prevent corrosion and store them in a dust-free environment. Adhering to these guidelines minimizes risks and maintains a safe working environment.
Maintaining electropolishing equipment is crucial for ensuring optimal performance and longevity. Regular maintenance includes several key practices:
By adhering to these practices, you can maintain your electropolishing equipment in excellent condition, ensuring consistent and high-quality results.
Yes, electropolishing can be used for other metals besides copper. This versatile electrochemical process is applicable to a wide range of metals, enhancing their surface finish and properties. Metals commonly electropolished include stainless steel (particularly the 300 and 400 series), aluminum, carbon steels, nickel alloys such as Inconel and Monel, titanium, and nitinol. Each metal requires specific electrolyte compositions and process parameters to achieve optimal results. Electropolishing improves corrosion resistance, provides a high-quality mirror-like finish, and facilitates ease of cleaning, making it valuable across various industries, including medical devices, aerospace, and automotive sectors.

