In the world of chemistry, few elements boast the versatility and industrial significance of nickel. This unassuming metal, found in everything from coins to batteries, owes much of its usefulness to its fascinating array of oxidation states. But what exactly are these states, and why are they crucial for nickel’s myriad applications? From the common Ni(0) and Ni(II) to the more elusive Ni(III) and Ni(IV), each oxidation state unlocks unique properties and reactions, impacting industries as diverse as catalysis and materials science. As we delve into the intricate dance of electrons that defines nickel’s chemical behavior, we’ll also explore how temperature can dramatically influence these oxidation processes. How does this subtle interplay affect the way nickel is harnessed in technology and industry? Join us on a technical deep dive into the oxidation states of nickel, where science meets everyday innovation.
Nickel is a transition metal, denoted by the symbol Ni and atomic number 28, recognized for its silvery-white appearance and high corrosion resistance. This element is valued for its excellent ductility, high melting point, and magnetic properties, making it indispensable in various industrial applications.
Nickel possesses several physical properties that make it valuable in industrial and engineering contexts:
Nickel’s versatility extends across numerous industries, from electronics to aerospace, due to its unique combination of properties.
One of nickel’s most critical industrial roles is in alloy formation. It combines with other metals to enhance their properties, resulting in materials like:
Nickel electroplating is a widespread process that involves coating a substrate with a thin layer of nickel. This enhances the substrate’s resistance to corrosion and wear, improves appearance, and provides electrical conductivity. Applications include:
Nickel is found in various everyday items, reflecting its broad application range:
Nickel’s role extends to the development of advanced materials for future technologies. Innovations include:
Nickel (Ni) is a transition metal with atomic number 28. It is known for its high melting point, magnetic properties, and excellent resistance to corrosion. Nickel is versatile because it can exist in different oxidation states and create many compounds.
Nickel forms several important compounds, each with distinct properties and applications. Some of the most notable nickel compounds include:
Nickel reacts with oxygen to form nickel oxides, which are significant in various industrial applications.
Nickel oxide (NiO) forms by heating nickel with oxygen at temperatures above 800°C, producing a stable oxide used in ceramics and batteries.
Under conditions of excess oxygen, nickel can form nickel(III) oxide (Ni₂O₃), although this compound is less common and less stable than NiO:
Nickel carbonyl (Ni(CO)₄) is a significant compound formed by the reaction of nickel with carbon monoxide. This reaction is critical in the Mond process for nickel purification:
Nickel carbonyl is very toxic and must be handled with care. It is used primarily in refining nickel to high purity levels.
Temperature greatly affects how nickel oxidizes. Elevated temperatures accelerate the formation of nickel oxides by increasing atomic movement and electron transfer rates. For instance, the formation of NiO requires temperatures above 800°C. Higher temperatures can also facilitate the formation of more complex oxides and nitrides.
Several factors affect the oxidation rate and behavior of nickel:
Understanding these factors is crucial for controlling nickel oxidation in various industrial processes and applications.
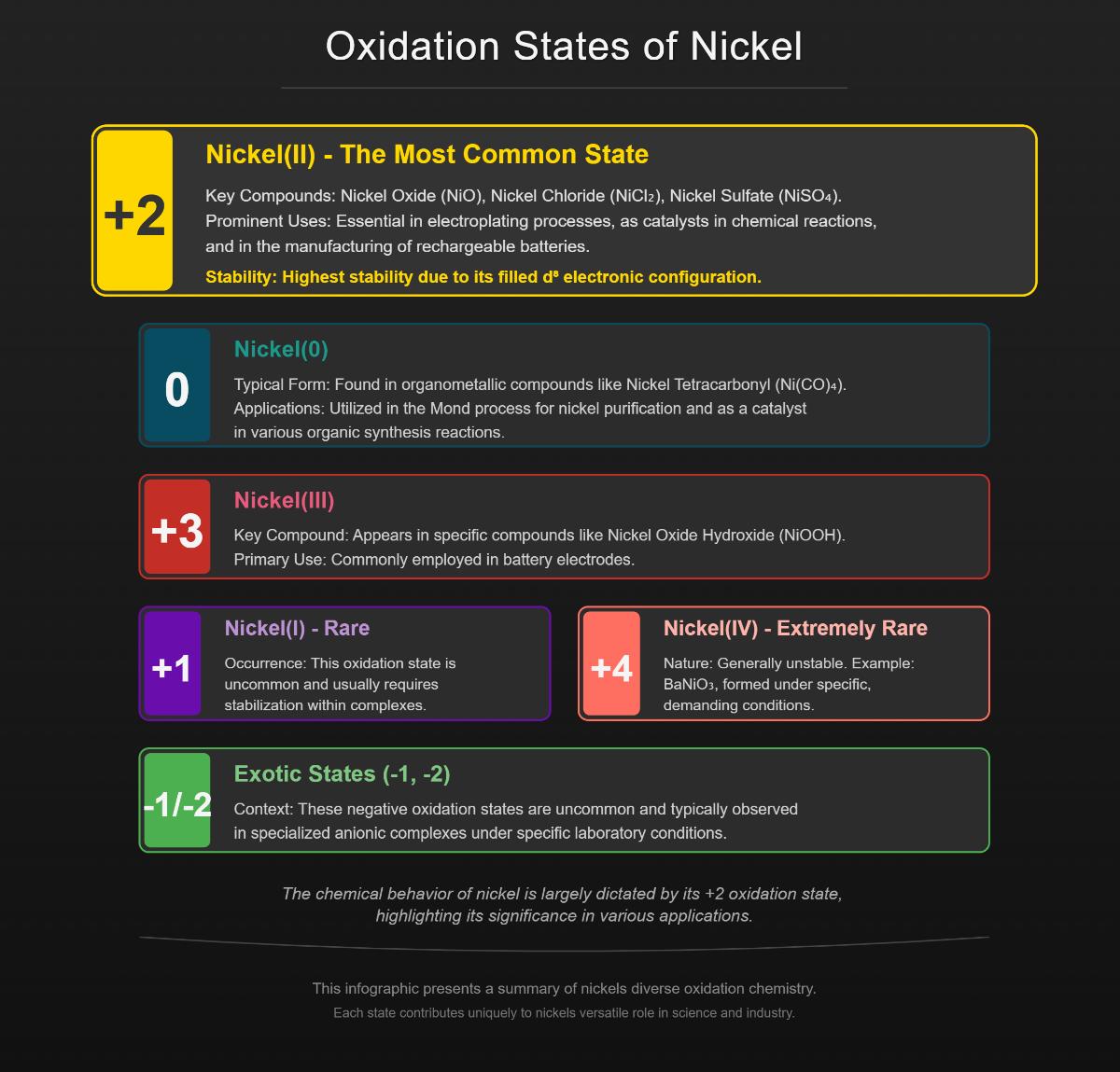
Oxidation states, also known as oxidation numbers, indicate the degree of oxidation of an atom within a compound, revealing how many electrons an atom gains, loses, or shares in chemical bonds. Nickel exhibits a range of oxidation states, from 0 to +4, each associated with distinct chemical properties and applications.
Nickel in the zero oxidation state, denoted as Ni(0), is found in compounds such as nickel tetracarbonyl (Ni(CO)₄). This volatile and highly toxic compound is utilized in the Mond process for refining nickel. These complexes, such as bis(cyclooctadiene)nickel(0), are catalysts in organonickel chemistry, facilitating a range of organic reactions.
Nickel with a +1 oxidation state, Ni(I), is relatively rare but significant in certain specialized complexes. For example, compounds like NiBr(PPh₃)₃ exhibit this oxidation state. In biological systems, Ni(I) plays a role in enzymes like [NiFe]-hydrogenase, which are involved in hydrogen production.
The +2 oxidation state, Ni(II), is the most stable and common form of nickel in compounds. Nickel(II) ions are found in a variety of compounds, such as nickel oxide (NiO), nickel chloride (NiCl₂), and nickel sulfate (NiSO₄). Ni(II) forms numerous complexes with different ligands, making it crucial in catalysis and various industrial processes. The versatility of Ni(II) compounds makes them essential for industrial and environmental applications.
Nickel in the +3 oxidation state, Ni(III), appears in compounds like nickel oxide hydroxide (NiOOH), which is used in rechargeable batteries. Although less common than Ni(II), Ni(III) is important in specific electrochemical applications.
Nickel with a +4 oxidation state, Ni(IV), is very rare and typically unstable. Limited compounds exhibit this state, such as BaNiO₃. Ni(IV) compounds are studied for their unique chemical properties, though they are not widely used in industrial applications due to their instability.
Nickel’s oxidation states significantly influence its reactivity and applications, such as its reaction with oxygen at high temperatures to form nickel oxides used in battery technology and catalysis. Nickel reacts with carbon monoxide to form nickel tetracarbonyl (Ni(CO)₄), a key compound in nickel purification. At high temperatures, nickel also reacts with nitrogen to form nickel nitride (Ni₃N₂), showcasing its versatility in forming different compounds under specific conditions. Understanding these chemical behaviors is essential for leveraging nickel’s properties in diverse industrial and technological contexts.
Nickel compounds are powerful catalysts that drive efficiency in industrial chemical processes. In their +2 oxidation state, compounds such as nickel oxide (NiO) and nickel sulfate (NiSO₄) significantly enhance reaction rates and lower energy consumption. Their versatility is evident in their widespread use:
Nickel compounds are essential in rechargeable batteries, where they help store and release energy efficiently. For example, nickel hydroxide (Ni(OH)₂) and nickel oxyhydroxide (NiOOH) are vital in:
The ability of nickel compounds to switch between +2 and +3 oxidation states ensures efficient charge-discharge cycles, crucial for battery performance.
In surface finishing, nickel salts are used in electroplating to create layers that protect metals from rust and wear. For instance, nickel chloride and nickel sulfate provide the nickel ions necessary for depositing durable nickel coatings. These coatings enhance corrosion resistance and longevity, benefiting industries like automotive, aerospace, and marine.
Nickel compounds also play a significant role in adding color and functionality to glass, ceramics, and pigments. For example, nickel oxide and nickel carbonates impart vibrant greens and blues to ceramics and glass, making them popular in artistic and decorative applications.
In alloy and steel production, nickel compounds serve as intermediates in producing high-quality nickel metal powders and refined nickel. Nickel sulfate and chloride are essential raw materials for electrorefining and electrodeposition processes. These high-performance nickel alloys are indispensable in aerospace engines, gas turbines, and power generation equipment, owing to their superior oxidation resistance and mechanical strength.
Nickel compounds enter the environment through a variety of pathways, both natural and human-made.
Nickel is widely used in various industrial processes, such as alloy production, electroplating, and battery manufacturing. These activities often release nickel into the environment through air emissions, wastewater, and solid waste. For instance, nickel dust is emitted during the refining and processing of nickel-containing ores.
Nickel is found in crude oil and coal, and burning these fuels releases nickel compounds into the air. This is a significant source of nickel emissions from power plants, industrial facilities, and vehicles.
Municipal and industrial waste, including electronic waste, can contribute to nickel pollution, especially when disposal and recycling practices are inadequate. Improper disposal leads to the leaching of nickel compounds into soil and groundwater.
Once released, nickel compounds interact with various environmental matrices, including air, water, and soil.
In the atmosphere, nickel compounds can attach to particulate matter, facilitating their transport over long distances. These particles eventually settle on the ground or are washed out by precipitation, leading to soil and water contamination.
In aquatic environments, nickel compounds can dissolve or bind to suspended particles and sediments. The solubility and mobility of nickel are influenced by water pH, with more acidic conditions increasing nickel solubility and bioavailability.
Nickel tends to accumulate in soils, particularly in areas near industrial sites. Soil pH and organic matter content significantly affect nickel’s mobility and bioavailability. In acidic soils, nickel is more likely to leach into groundwater, while in alkaline soils, it tends to remain bound to soil particles.
Exposure to nickel compounds can pose various health risks to humans, including skin allergies, respiratory issues, and cancer.
Nickel dermatitis is a common allergic reaction resulting from direct skin contact with nickel-containing items. This condition is characterized by itching, redness, and rashes, particularly in individuals with nickel sensitivity.
Inhalation of nickel compounds, especially in occupational settings, can lead to respiratory problems, including chronic bronchitis and reduced lung function. Workers in nickel refining, electroplating, and welding industries are at higher risk.
Certain nickel compounds, such as nickel refinery dust and nickel subsulfide, are classified as human carcinogens. Long-term exposure to these compounds has been linked to an increased risk of lung and nasal cancers.
Nickel compounds affect ecosystems and wildlife, with varying degrees of toxicity depending on the compound’s form and concentration.
Nickel compounds can accumulate in plants and animals, leading to bioaccumulation in the food chain and causing toxic effects such as reproductive and developmental issues in wildlife.
Nickel contamination in soil can hinder plant growth and reduce soil fertility. In aquatic environments, elevated nickel levels can be toxic to fish and other aquatic organisms, disrupting ecosystems and biodiversity.
Addressing the environmental impact of nickel compounds involves several strategies aimed at reducing emissions and promoting sustainable practices.
Improving the recycling of nickel-containing products, such as batteries and electronic waste, can significantly reduce the release of nickel compounds into the environment. Efficient recycling processes recover valuable nickel, minimizing waste and conserving natural resources.
Adopting cleaner production technologies and practices in nickel-related industries can lower emissions. For example, implementing advanced filtration and scrubber systems can reduce airborne nickel emissions from industrial processes.
Strengthening regulations on the use, disposal, and management of nickel compounds is crucial for protecting human health and the environment. Regulatory measures may include setting limits on nickel emissions, monitoring environmental concentrations, and enforcing proper waste management practices.
Below are answers to some frequently asked questions:
Nickel exhibits several oxidation states, with the most common being +2 (Nickel(II)). Nickel(II) forms stable compounds such as nickel oxide (NiO), nickel chloride (NiCl₂), and nickel sulfate (NiSO₄). These compounds are widely used in applications like electroplating, catalysts, and rechargeable batteries.
Nickel can also exist in the 0 oxidation state, typically found in organometallic compounds like nickel tetracarbonyl (Ni(CO)₄), which is used in the Mond process for nickel purification and in catalytic roles in organic synthesis.
The +1 oxidation state of nickel is rare and usually stabilized in complexes, while the +3 oxidation state appears in specific compounds like nickel oxide hydroxide (NiOOH), used in battery electrodes. The +4 oxidation state is extremely rare and generally unstable, appearing in compounds like BaNiO₃ under specific conditions.
Additionally, exotic oxidation states such as -1 and -2 can be observed in specialized anionic complexes, though these are uncommon. The stability of these oxidation states varies, with Ni(II) being the most stable due to its filled d⁸ electronic configuration.
Temperature significantly affects the oxidation of nickel by influencing both the rate and mechanisms involved. At moderate temperatures (250°C to 500°C), nickel oxidation is primarily diffusion-controlled, meaning the rate is determined by ion movement through the oxide layer rather than surface reactions. As temperatures increase (500°C to 1400°C), the properties of the oxide layer and metal-oxide interactions change, potentially altering oxidation mechanisms.
High temperatures, such as 1000°C, can couple oxidation with deformation processes, impacting nickel’s mechanical properties. Additionally, the presence of elements like chromium in nickel alloys can modify oxidation rates and stability, with chromium enhancing oxidation resistance through complex phase interactions. Understanding these temperature effects is crucial for industrial applications of nickel and its alloys, where oxidation behavior can influence material performance and longevity.
Nickel oxide is a versatile material with numerous practical applications across various industries. In electronics, it is used to manufacture thermistors, varistors, resistors, capacitors, and potentiometers, which are essential for temperature sensing, voltage regulation, and electrical resistance adjustment. In energy storage and conversion, nickel oxide plays a crucial role in lithium-ion and alkaline batteries, as well as in solid oxide fuel cells. Its catalytic properties are employed in automotive emission reduction and methane reforming processes. Nickel oxide’s optical characteristics make it suitable for smart windows and gas sensors. Additionally, it finds use in biomedical applications, such as drug delivery and diagnostics, and is integral in creating refractory materials and glass ceramics. Its role in metallurgical processes as an alloying component further underscores its importance in modern technology and industry.
Nickel is widely used in catalysis due to its ability to adopt multiple oxidation states, including Ni(0), Ni(I), Ni(II), and Ni(III). This versatility enables nickel to participate in various catalytic processes, such as oxidative addition and reductive elimination, which are crucial in forming and breaking chemical bonds. For instance, in the hydrogenation of alkenes and alkynes, nickel catalysts facilitate the addition of hydrogen to these unsaturated compounds, converting them into saturated hydrocarbons. Nickel is also pivotal in cross-coupling reactions, which form carbon-carbon bonds essential in organic synthesis. Additionally, nickel’s involvement in single-electron transfer processes and radical pathways enables unique transformations that are less common with noble metal catalysts. This makes nickel an invaluable component in both industrial applications, such as petroleum refining and hydrogen production, and in sustainable chemistry, including CO2 functionalization.
Nickel(II) and nickel(III) compounds differ primarily in their oxidation states, which significantly influence their chemical behavior, stability, and applications. Nickel(II) compounds, with a +2 oxidation state, are the most stable and common. They form well-characterized compounds like nickel(II) oxide (NiO) and nickel(II) sulfate (NiSO₄), often adopting octahedral coordination geometries. These compounds are widely used in industrial processes such as catalysis and electroplating due to their high stability and moderate reactivity.
In contrast, nickel(III) compounds, with a +3 oxidation state, are less common and generally less stable. They are strong oxidizing agents, exemplified by nickel(III) oxide (Ni₂O₃), which is used in rechargeable battery technology. Nickel(III) compounds typically exhibit shorter metal-ligand bond lengths and are more reactive, making them important in redox chemistry and catalysis. The Ni(III)/Ni(II) redox couple is particularly significant in both synthetic and biological systems.

