Imagine a reaction so dynamic that it transforms an everyday metal into a source of clean energy. When aluminum comes into contact with sodium hydroxide, a fascinating chemical process unfolds, breaking down the aluminum and releasing hydrogen gas—a key player in sustainable energy solutions. But how does this reaction work, and what makes it so significant? From the generation of sodium aluminate to the bubbling release of hydrogen, the process is both chemically intriguing and practically impactful. In this article, we’ll take you step-by-step through the reaction mechanism, explore its practical applications, and provide essential safety guidelines. Ready to uncover how science turns aluminum into a tool for innovation? Let’s dive in!
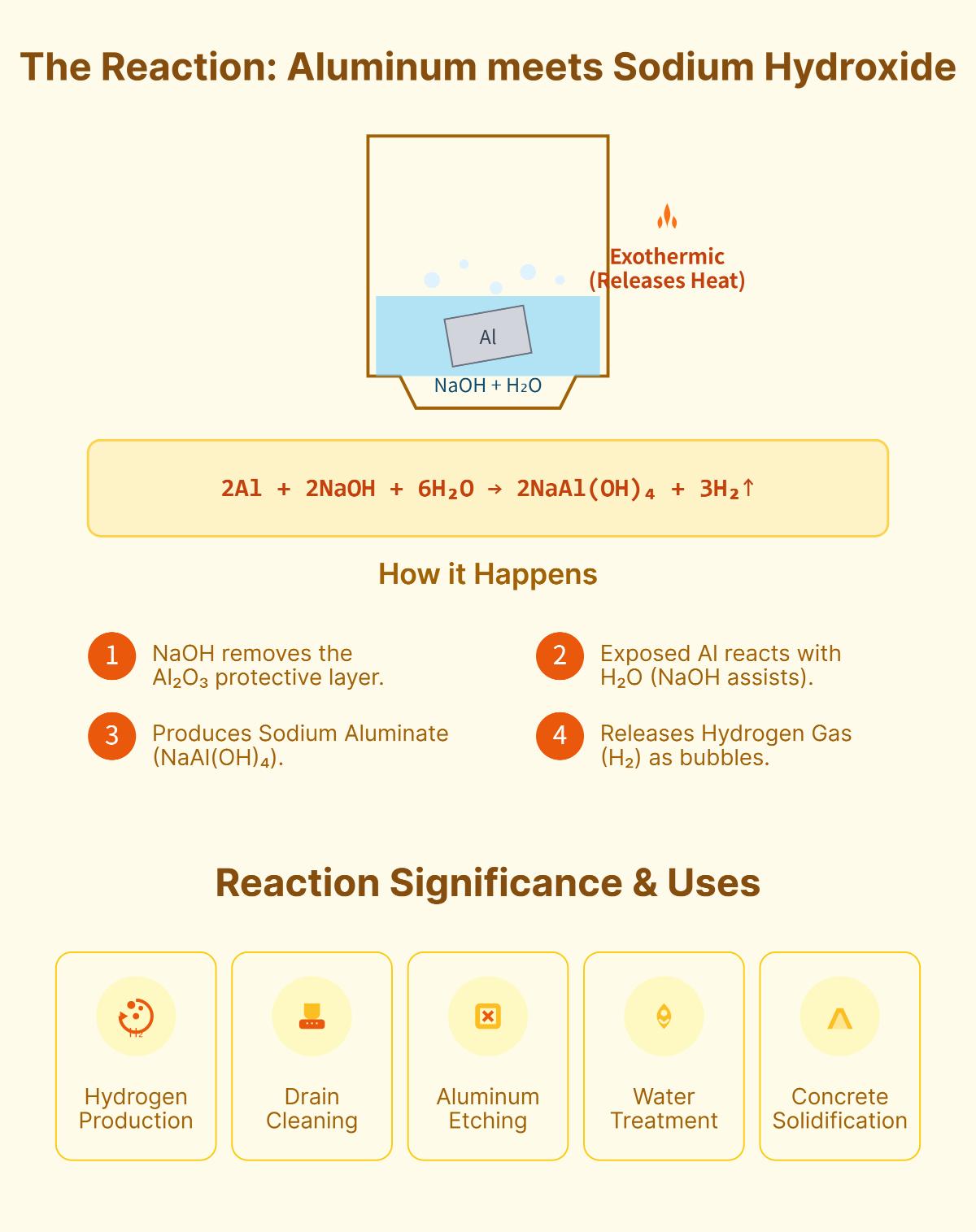
Aluminum and sodium hydroxide interact in a fascinating chemical reaction that has significant industrial and scientific implications. Aluminum is a lightweight, silvery-white metal known for its excellent conductivity, corrosion resistance, and high strength-to-weight ratio, while sodium hydroxide, commonly referred to as caustic soda or lye, is a highly caustic base used in various industrial processes.
The chemical reaction between aluminum and sodium hydroxide is notable for several reasons. This reaction releases a lot of heat, making it highly exothermic. Additionally, it produces hydrogen gas, which has considerable implications for energy production and storage.
Hydrogen gas is a clean fuel source that produces only water when used in fuel cells, making it environmentally friendly and important for sustainable energy solutions. This makes the reaction between aluminum and sodium hydroxide particularly interesting in the context of the hydrogen economy, where sustainable and efficient hydrogen production methods are highly sought after.
Additionally, the reaction forms sodium aluminate, a compound with various industrial applications. Sodium aluminate is used in water treatment, the paper industry, and as an additive in concrete. Understanding this reaction is valuable for both fundamental chemistry knowledge and practical industrial applications.
Let’s explore how aluminum and sodium hydroxide interact to produce hydrogen gas and sodium aluminate.
The balanced chemical equation representing the reaction is:
This equation shows that two moles of aluminum react with two moles of sodium hydroxide and six moles of water to produce two moles of sodium aluminate and three moles of hydrogen gas.
Oxide Layer Removal: Aluminum naturally forms a thin oxide layer (Al2O3) that protects it from further reactions. Sodium hydroxide (NaOH) dissolves this protective layer, exposing the aluminum metal underneath. The dissolution can be represented as:
Surface Activation: After the oxide layer is removed, the aluminum can react with sodium hydroxide and water.
Understanding this reaction helps us see how redox reactions work and their uses in technology.
To perform the reaction between aluminum and sodium hydroxide, you will need the following materials and equipment:
When performing the reaction between aluminum and sodium hydroxide, it is crucial to follow strict safety guidelines to prevent accidents and ensure a safe working environment. The following safety measures are essential for handling the chemicals and conducting the experiment safely.
Adhering to these safety precautions will help ensure a safe and controlled environment when conducting the reaction between aluminum and sodium hydroxide. Always prioritize safety and follow best practices to minimize risks.
The reaction between aluminum and sodium hydroxide offers an efficient way to produce hydrogen gas, a clean and renewable energy source. Hydrogen gas generated from this reaction can be used in fuel cells to produce electricity, emitting only water as a byproduct. This positions the reaction as a potential contributor to the hydrogen economy, where sustainable energy solutions are a priority. Producing hydrogen on-demand with easily accessible aluminum and sodium hydroxide makes this process ideal for portable and remote applications.
The aluminum-sodium hydroxide reaction plays a critical role in metal surface treatment processes such as etching and anodizing. Sodium hydroxide effectively removes the natural oxide layer on aluminum surfaces, exposing the underlying metal for further treatment. This reaction is widely employed in industries requiring precise surface preparation, such as aerospace and automotive manufacturing. Additionally, the resulting sodium aluminate can contribute to the formation of durable aluminum oxide layers during anodizing, enhancing corrosion resistance and surface hardness.
Sodium aluminate, a byproduct of the reaction, is extensively used in water treatment processes. It acts as a coagulant, aiding in the removal of impurities and suspended particles from water. This is especially useful in municipal and industrial wastewater treatment, where water quality is essential. The reaction’s ability to generate sodium aluminate on-site adds convenience and cost-effectiveness to water treatment operations.
Sodium aluminate from the reaction is used in construction as a cement and concrete additive. It acts as an accelerator, reducing setting times and improving early strength development in concrete mixtures. This property is especially beneficial in cold weather conditions or in projects requiring rapid construction timelines. The dual benefit of producing hydrogen gas and a useful construction material makes this reaction advantageous for integrated industrial applications.
This reaction provides a sustainable way to produce energy and recycle materials. Aluminum, often derived from recycled sources such as beverage cans, provides a low-cost and abundant raw material for hydrogen generation. This aligns with global efforts to promote circular economies and reduce waste. Furthermore, the exothermic nature of the reaction can provide additional energy for industrial processes, enhancing overall energy efficiency.
The reaction is economically viable in scenarios where aluminum waste can be repurposed, reducing raw material costs. Additionally, the simple setup and straightforward execution of the reaction make it accessible for small-scale and decentralized hydrogen production. This has potential applications in emergency power systems, off-grid energy solutions, and even educational demonstrations to promote awareness of sustainable energy technologies.
Below are answers to some frequently asked questions:
The chemical reaction between aluminum (Al) and sodium hydroxide (NaOH) is a notable exothermic process that results in the formation of sodium aluminate (NaAl(OH)4) and hydrogen gas (H2). The balanced chemical equation for this reaction is:
Initially, sodium hydroxide dissolves the aluminum oxide (Al2O3) layer on the aluminum’s surface, exposing the metal for further reaction. Subsequently, aluminum interacts with water and sodium hydroxide to produce sodium aluminate and hydrogen gas. Sodium hydroxide plays a crucial role in both removing the oxide layer and stabilizing aluminum ions, facilitating their conversion into aluminate ions. This reaction is significant for hydrogen production and various industrial applications, such as drain cleaning, aluminum etching, water treatment, and concrete solidification.
The reaction between aluminum and sodium hydroxide produces hydrogen gas through a series of steps. Initially, sodium hydroxide dissolves the protective aluminum oxide layer on the aluminum surface. This exposes the aluminum metal, allowing it to react with water and sodium hydroxide.
In this process, aluminum reacts with water in the presence of sodium hydroxide, forming sodium aluminate and releasing hydrogen gas. Sodium hydroxide plays a crucial role as it facilitates the dissolution of the oxide layer and stabilizes the aluminum ions, thereby promoting the reaction. The hydrogen gas is produced as a byproduct, which can be observed as bubbles forming in the solution. This reaction is highly exothermic, meaning it releases a significant amount of heat, further driving the production of hydrogen gas.
The chemical reaction between aluminum (Al) and sodium hydroxide (NaOH) produces two primary products: sodium aluminate (NaAl(OH)₄) and hydrogen gas (H₂). When aluminum reacts with sodium hydroxide in the presence of water, it undergoes a redox reaction, which can be represented by the balanced chemical equation:
Sodium aluminate is an important compound used in various industrial processes, including water treatment and construction. It is soluble in water and helps accelerate concrete solidification, especially in cold environments. Hydrogen gas, the other product, is highly flammable and has significant potential in hydrogen fuel technologies, making this reaction valuable for hydrogen production.
When handling sodium hydroxide (NaOH), several safety precautions are essential due to its highly corrosive nature. Personal protective equipment (PPE) is crucial: wear latex or nitrile gloves, long sleeves, pants, and a chemical-resistant suit to prevent skin contact. Eye protection is necessary, using safety glasses with side shields or goggles, and a face shield for additional safety. If there’s a risk of inhaling vapors, use a respirator.
Store NaOH in a cool, dry, well-ventilated area, away from incompatible materials like water, acids, and metals. Always keep it in tightly closed, original containers. When diluting, add NaOH slowly to cold water while stirring to avoid violent reactions. Never ingest or inhale NaOH, and wash hands thoroughly after handling. In case of skin contact, flush the area with water for 15 minutes; for eye contact, flush eyes and seek medical attention. If inhaled, move to fresh air and provide oxygen if needed; if ingested, rinse the mouth and seek medical help immediately. Following these guidelines ensures safe handling of sodium hydroxide.
Yes, the reaction between aluminum and sodium hydroxide has several practical applications. The production of hydrogen gas through this reaction is valuable for hydrogen fuel technologies, such as fuel cells for generators and vehicles, which offer cleaner energy solutions. Additionally, the reaction is used in aluminum etching and anodizing, where sodium hydroxide helps dissolve the existing oxide layer on aluminum surfaces, enhancing corrosion resistance and durability. Sodium aluminate, a byproduct of the reaction, is employed in water treatment to remove impurities and in the construction industry to accelerate concrete solidification. These diverse applications highlight the industrial significance and potential future technological uses of this chemical reaction.
Aluminum’s economic viability for hydrogen production hinges on overcoming several challenges. The reaction between aluminum and sodium hydroxide produces hydrogen gas, as shown by the equation (2Al + 2NaOH + 6H_2O → 2NaAl(OH)_4 + 3H_2). While this reaction is thermodynamically favorable, the cost of aluminum metal and the process’s efficiency are significant hurdles. Currently, hydrogen production from aluminum is not cost-competitive with other methods due to the high cost of aluminum and the need to prevent the formation of a protective oxide layer that hinders the reaction. Additionally, recycling aluminum efficiently and scaling up production processes are critical to making this method economically viable. Further technological advancements and research are necessary to enhance efficiency and reduce costs, making aluminum a feasible option for hydrogen production in the future.

