In the world of electroplating, the choice between tin and silver plating can significantly impact a product’s performance. But what exactly sets these two apart? Tin plating is known for its good solderability, while silver plating offers excellent corrosion resistance. As an intermediate-level enthusiast or professional, understanding these differences is crucial. This article will delve into their processes, characteristics, and real – world applications. So, are you ready to discover which plating is the best fit for your specific needs?
Electroplating is a metallurgical process that involves the deposition of a thin layer of metal onto the surface of a conductive material using an electric current. This technique improves the strength and appearance of metals, enhancing their performance in various applications, from electronics to aerospace.
The electroplating process involves several key steps:
Tin plating involves depositing a layer of tin onto a substrate to enhance its corrosion resistance, solderability, and appearance. This process is commonly used in the electronics industry for components such as connectors and circuit boards, as well as in the food industry for packaging materials to prevent rust and contamination. Tin plating can be achieved through various methods, including electroplating and hot-dipping, each offering different benefits depending on the application requirements.
Silver plating is used to impart superior electrical and thermal conductivity to the substrate. Silver’s excellent conductivity makes it ideal for high-performance applications, including high-frequency electronics and precision instruments. Additionally, silver plating is favored for its aesthetic appeal and durability, making it suitable for decorative items and jewelry.
Tin plating involves coating a metal with a thin layer of tin to enhance its properties, such as corrosion resistance and solderability. This electroplating process enhances the substrate’s properties, particularly for applications requiring improved corrosion resistance, solderability, and a non-toxic finish. The process typically includes cleaning the substrate, immersing it in an electrolyte solution containing tin ions, and applying an electric current to deposit the tin onto the substrate.
Tin plating is highly valued for its corrosion resistance, as the tin layer forms a stable oxide film that protects the underlying metal from environmental damage. This characteristic makes tin plating suitable for applications in harsh environments, such as marine settings and high-moisture conditions.
Tin’s low melting point and strong bonding capabilities make it ideal for soldering, ensuring reliable joints and stable electrical connections in electronics. The tin layer facilitates reliable soldered joints and stable electrical connections, which are crucial in electronics manufacturing. This property ensures that components can be easily and securely joined, enhancing the overall durability and performance of electronic devices.
Compared to more expensive metals like gold and silver, tin is relatively inexpensive. This cost advantage makes tin plating a popular choice for large-scale manufacturing where budget constraints are significant. The affordability of tin plating does not compromise its effectiveness, providing a practical solution for various industrial applications.
In the electronics industry, tin plating is extensively used to enhance the performance and reliability of components. It is commonly applied to connectors, terminals, and printed circuit boards (PCBs). The excellent solderability and corrosion resistance of tin plating ensure stable electrical connections and long-lasting performance of electronic devices.
Tin plating is widely used in the food packaging industry due to its non-toxic nature and ability to prevent corrosion. Tin-plated steel, commonly known as tinplate, is used to manufacture cans and containers for food and beverages. The tin layer protects the contents from contamination and extends the shelf life of the products.
In the aerospace industry, tin plating is used to protect various components from corrosion and wear. The lightweight nature of tin, combined with its protective properties, makes it suitable for coating critical parts such as fasteners, connectors, and avionics components. Tin plating ensures the longevity and reliability of these components in demanding aerospace environments.
Hot-dipping involves submerging the metal into molten tin, creating a thick, durable coating that offers excellent corrosion protection. This method produces a thick, durable coating that offers excellent protection against corrosion. Hot-dipped tin plating is commonly used for producing tinplate for cans and other containers.
Electroplating is a precise method that uses an electric current to deposit tin ions onto the metal surface. This process allows for controlled thickness and uniform coverage, making it ideal for applications requiring specific coating properties. Electroplating is widely used in the electronics industry for coating connectors and PCBs.
Chemical tin plating, also known as immersion tin plating, involves a displacement reaction to deposit tin onto copper surfaces without using an electric current. This method is particularly useful for coating PCBs and other small components where precision and cost-effectiveness are important.
Silver plating involves coating another metal with a thin layer of silver. This is done by first cleaning the base metal to remove any impurities. The clean metal is then dipped into an electrolyte solution that contains silver ions. An electric current is applied, causing the silver ions to move to the base metal and form a uniform layer of silver.
Silver is the most conductive metal, which makes it invaluable in applications requiring high electrical and thermal conductivity. For example, in high-frequency electronics like telecommunications equipment and advanced computing systems, silver plating minimizes energy loss and heat generation. This is crucial for maintaining signal integrity and performance. In comparison, while tin also conducts electricity, it does not match silver’s efficiency, making it less suitable for these high-demand applications.
Silver can resist oxidation but is prone to tarnishing due to its reaction with sulfur compounds in the air. This forms a layer of silver sulfide, which can affect its appearance and functionality. To counteract this, additional treatments such as anti-tarnish coatings and lacquers can be applied to silver-plated items to enhance their corrosion resistance. These treatments help maintain the integrity and appearance of the silver layer. On the other hand, tin naturally forms a stable oxide layer that provides excellent corrosion protection, especially in moist environments.
Silver plating is prized for its bright, mirror-like finish, making it ideal for decorative applications such as jewelry and luxury items. This lustrous appearance enhances the visual appeal and value of the products. Tin plating, however, offers a more subdued, matte finish and is less commonly used where aesthetics are a primary concern.
In high-frequency electronics, the superior conductivity of silver is essential. For instance, silver-plated connectors and contacts in telecommunications equipment ensure minimal signal loss and efficient performance. This is critical for maintaining the reliability and speed of data transmission. While tin-plated components are used in various electronic devices, they are not as effective in high-frequency applications due to their lower conductivity.
The aerospace industry and precision instruments demand materials that perform reliably under extreme conditions. Silver plating is ideal here due to its excellent electrical and thermal properties, as well as its resistance to oxidation at high temperatures. This makes it suitable for components like connectors, bearings, and switches that need to operate flawlessly. Tin plating, while used for its corrosion resistance, does not offer the same level of performance in these critical applications.
The aesthetic qualities of silver plating make it a popular choice for jewelry and decorative items. Its bright, reflective finish adds elegance and sophistication, enhancing the overall appearance of pieces such as necklaces, bracelets, earrings, and ornamental objects. Silver-plated items are often more affordable than their solid silver counterparts, making them accessible to a wider range of consumers.
Corrosion resistance is a material’s ability to resist damage from chemical reactions with its environment, such as oxidation and rusting. In metal plating, this property is crucial as it protects the underlying substrate from degradation, ensuring the longevity and performance of the metal component.
Tin plating offers a fair level of corrosion protection. When tin oxidizes, it creates a protective layer that prevents further corrosion. This characteristic makes tin plating suitable for applications in moderately corrosive environments, like general-purpose electronics and food packaging. However, tin is more reactive with oxygen compared to silver. In high humidity or highly corrosive environments, the oxide layer can degrade, reducing conductivity and corrosion resistance. Also, tin’s relatively soft nature can be a drawback in high-wear situations, as the protective layer can wear away, exposing the substrate to corrosion.
Silver plating is well-known for its excellent corrosion resistance. Silver has a weak reactivity with oxygen and moisture, allowing silver-plated surfaces to maintain their integrity over long periods, especially in environments with regular air and contaminant exposure. While silver can tarnish from sulfur or hydrogen sulfide, it generally offers better protection against oxidation than tin. This makes silver plating ideal for high-performance applications where long-term corrosion resistance is essential, such as in high-frequency electronics and precision instruments.
Solderability refers to the ease with which a metal surface can be soldered to form a strong, reliable electrical and mechanical connection. This property is crucial in electronics manufacturing where secure bonds are necessary for optimal component performance. The solderability of a plated surface depends on various factors, including the plating material, surface preparation, and environmental conditions.
Tin plating is renowned for its excellent solderability, making it a preferred choice in many electronic applications.
Silver plating is highly valued for its superior solderability, particularly in high-performance and precision applications.
When comparing tin and silver plating for soldering applications, several key factors come into play:
Tin and silver plating are essential in the electronics industry, each offering unique advantages for different applications.
Tin plating is extensively used for its excellent solderability, making it ideal for connectors, terminals, and printed circuit boards (PCBs), while its corrosion resistance ensures long-term reliability of electronic components. Additionally, the cost-effectiveness of tin plating makes it a popular choice for mass production in consumer electronics.
Silver plating, known for its superior electrical conductivity, is preferred for high-performance electronic applications, such as connectors and contacts where minimal signal loss is critical. It is commonly used in telecommunications equipment and high-speed data transmission lines. The enhanced conductivity of silver plating ensures optimal performance in demanding electronic environments.
The aerospace industry demands materials that can withstand extreme conditions, making both tin and silver plating valuable for different reasons.
In the aerospace industry, tin plating is valued for its excellent corrosion resistance and cost-effectiveness. It is particularly useful for coating fasteners, connectors, and other components that require protection from harsh environments. The lightweight nature of tin also contributes to its suitability in aerospace applications.
Silver plating is favored in aerospace applications that require high conductivity and resistance to oxidation. Components such as connectors, switches, and bearings benefit from silver plating’s superior electrical and thermal properties. The durability and reliability of silver plating make it indispensable for critical aerospace systems.
Both tin and silver plating play significant roles in the automotive industry, enhancing the performance and longevity of various components.
Tin plating is widely used in the automotive industry for its corrosion resistance and solderability, making it ideal for electrical connectors, terminals, and battery components. This ensures reliable electrical connections and protection against environmental factors. The affordability of tin plating also makes it suitable for large-scale automotive manufacturing.
Silver plating is used in high-performance automotive applications where superior conductivity is required. It is often applied to sensors, connectors, and other components involved in electronic control systems. The excellent electrical properties of silver plating enhance the efficiency and reliability of advanced automotive technologies.
Tin plating is particularly prominent in the food packaging industry due to its non-toxic nature and corrosion resistance.
Tin plating is used to produce tinplate, which is commonly used for manufacturing cans and containers for food and beverages. The tin layer prevents contamination and extends the shelf life of the products by protecting them from corrosion. This makes tin plating a crucial component in ensuring the safety and quality of packaged food.
Silver plating is not typically used in food packaging due to its higher cost and the specific requirements of the industry. However, it may find niche applications in high-end food storage solutions where its antimicrobial properties and aesthetic appeal are valued.
Both tin and silver plating continue to evolve with advancements in technology and industry demands.
Innovations in tin plating include its application in fuel cell technology and lightweight structural components. These advancements enhance the reliability and efficiency of tin-plated components in the aerospace and automotive sectors.
Silver plating remains a choice for high-performance applications, especially where conductivity and aesthetics are critical. Future trends may see its expanded use in advanced computing and telecommunications, where the demand for superior electrical performance continues to grow.
Tin plating is known for its cost-effectiveness, making it a popular choice in various industrial applications. Several factors contribute to the lower cost of tin plating. Firstly, tin is more abundant and easier to extract than silver, which significantly reduces raw material costs.
Tin is relatively abundant and inexpensive compared to precious metals like silver, which significantly reduces the overall cost of production for items that use tin. This makes tin an attractive option for manufacturers who need a cost-effective material without compromising on quality.
The process of tin electroplating is relatively straightforward and less complex than that of silver plating. This simplicity leads to lower operational costs, including less energy use and shorter processing times. The ease of the tin plating process allows for high throughput, which is beneficial for mass production.
Tin-plated surfaces resist corrosion well, which means less frequent maintenance and replacements, thus lowering long-term costs. This durability helps in reducing the total cost of ownership over the lifespan of the plated components.
Silver plating, while offering superior properties in certain applications, is generally more expensive. The higher cost is influenced by several critical factors:
Silver is a precious metal with a higher market value compared to tin. The cost of silver is subject to fluctuations based on market demand and supply, contributing to the overall expense of products that utilize silver in their manufacturing. Tin, on the other hand, is generally more stable in price but can still be affected by factors such as mining output and geopolitical events.
The silver plating process is more complex and requires strict safety measures. The handling and disposal of silver solutions must comply with environmental regulations, adding to the operational costs. Additionally, the need for specialized equipment and skilled labor further increases the expense.
The superior electrical and thermal conductivity of silver justifies its higher cost in high-performance applications. The enhanced properties of silver plating, such as its excellent conductivity and aesthetic appeal, make it indispensable for precision instruments, high-frequency electronics, and decorative items.
| Aspect | Tin Plating | Silver Plating |
|---|---|---|
| Material Cost | Lower | Higher |
| Process Complexity | Simpler | More complex |
| Operational Costs | Lower | Higher |
| Maintenance | Low frequency | Moderate frequency |
Tin plating is more cost-effective for applications where budget constraints are significant and the superior properties of silver are not required. It is ideal for general electronics, automotive parts, and food packaging due to its affordability and adequate performance.
Tin plating is widely used due to its affordability and functional benefits, but it also presents several environmental considerations.
The electroplating process for tin involves significant energy usage and generates hazardous waste. Effective management of these byproducts is essential to prevent environmental contamination. Despite these challenges, tin is less toxic than some alternative metals like cadmium and chromium, which makes it a relatively safer option.
Tin is more abundant and less expensive than precious metals like silver. Its abundance makes it a cost-effective choice for large-scale industrial applications.
Effective waste treatment and recycling practices, such as robust systems for electrolyte solutions and other waste products, are essential to reduce the environmental footprint of tin plating.
Silver plating, known for its superior conductivity, also has distinct environmental implications.
Silver is a precious metal, more expensive and less abundant than tin. This scarcity impacts its economic sustainability, making it less accessible for cost-sensitive projects. The high market value of silver can drive over-extraction, leading to environmental degradation in mining areas.
While silver is less toxic than some heavy metals, its extraction and refining processes can have significant environmental impacts. The high conductivity of silver can reduce energy losses in high-performance applications, potentially offsetting some of the environmental costs associated with its production.
Silver’s excellent electrical and thermal conductivity allows components to operate more efficiently, potentially reducing energy consumption in their applications. This efficiency can be particularly beneficial in high-frequency electronics where energy savings are critical.
| Criteria | Tin Plating | Silver Plating |
|---|---|---|
| Material Cost and Availability | Less expensive; widely available. | More expensive; less abundant. |
| Environmental Impact | Generates hazardous waste; requires careful management. | High material value may lead to over-extraction; requires energy-efficient use. |
| Energy Efficiency | Provides moderate energy efficiency. | Offers high energy efficiency due to superior conductivity. |
| Resource Sustainability | More economically sustainable due to abundance. | Less economically sustainable due to scarcity and high cost. |
| Toxicity and Safety | Less toxic than heavy metals like cadmium. | Less toxic but high market value can lead to environmental concerns. |
Choosing between tin and silver plating involves balancing performance needs with sustainability considerations. Tin plating is generally more cost-effective and environmentally friendly, requiring careful waste management to mitigate its environmental impact. Silver plating, while offering superior performance, comes with higher material costs and potential environmental impacts from extraction. The decision ultimately depends on the specific application requirements and the prioritization of sustainability goals.
In the electronics industry, tin plating is widely used to improve the performance and reliability of components like connectors, terminals, and printed circuit boards (PCBs). Companies producing consumer electronics often utilize tin-plated connectors to ensure stable and durable electrical connections. The excellent solderability of tin makes it ideal for these applications, facilitating the efficient assembly of electronic devices.
In the food packaging industry, tin plating is applied to steel to produce tinplate, which is used to manufacture cans and containers for food and beverages. The tin layer prevents corrosion and contamination, ensuring the safety and longevity of packaged foods. Major food packaging companies rely on tin plating to meet safety standards and extend the shelf life of their products.
Silver plating is crucial in the production of high-frequency electronic components, such as connectors and RF (radio frequency) connectors. These components benefit from silver’s excellent electrical conductivity, minimizing signal loss and ensuring efficient performance. Telecommunication companies often use silver-plated connectors in their equipment to maintain high signal integrity and reliability.
In the aerospace industry, silver plating is used for parts that require high conductivity and resistance to oxidation. For example, silver-plated connectors and switches are essential in aircraft systems, where reliable performance under extreme conditions is critical. Aerospace manufacturers prefer silver plating for these components to ensure their durability and functionality over extended periods.
Experts highlight that the choice between tin and silver plating often comes down to cost and performance requirements. Tin plating is more cost-effective and suitable for budget-conscious applications like mass-producing consumer electronics. On the other hand, silver plating, despite its higher cost, is chosen for applications requiring superior conductivity and performance, justifying the investment in high-frequency electronics and precision instruments.
Industry experts note that for applications involving high-frequency signals or requiring minimal energy loss, silver plating is the preferred choice due to its unparalleled conductivity. However, for general electronics and applications where cost is a major factor, tin plating offers a practical and efficient solution. The decision is often influenced by the specific needs of the application, balancing cost against performance benefits.
For electronics and telecommunications, experts recommend using tin plating for components where solderability and cost are primary concerns. Tin plating ensures reliable connections and is cost-effective for large-scale production. However, for high-frequency applications, silver plating is advised due to its superior conductivity and minimal signal loss.
For aerospace and automotive industries, where durability and extreme performance are crucial, experts recommend silver plating for components like connectors and switches. Silver’s resistance to oxidation and high conductivity make it ideal for these demanding applications. Conversely, tin plating can be used for components that require corrosion resistance and where cost is a significant factor.
For food packaging, experts advocate for tin plating due to its non-toxic nature and corrosion resistance. Tin-plated steel, or tinplate, is essential for ensuring the safety and longevity of canned foods and beverages. The affordability and effectiveness of tin plating make it the go-to choice in this industry.
Selecting the appropriate plating method involves considering the specific requirements of the application, including cost, performance, and environmental conditions. By understanding the strengths and limitations of tin and silver plating, manufacturers can make informed decisions that optimize both performance and cost-efficiency.
Below are answers to some frequently asked questions:
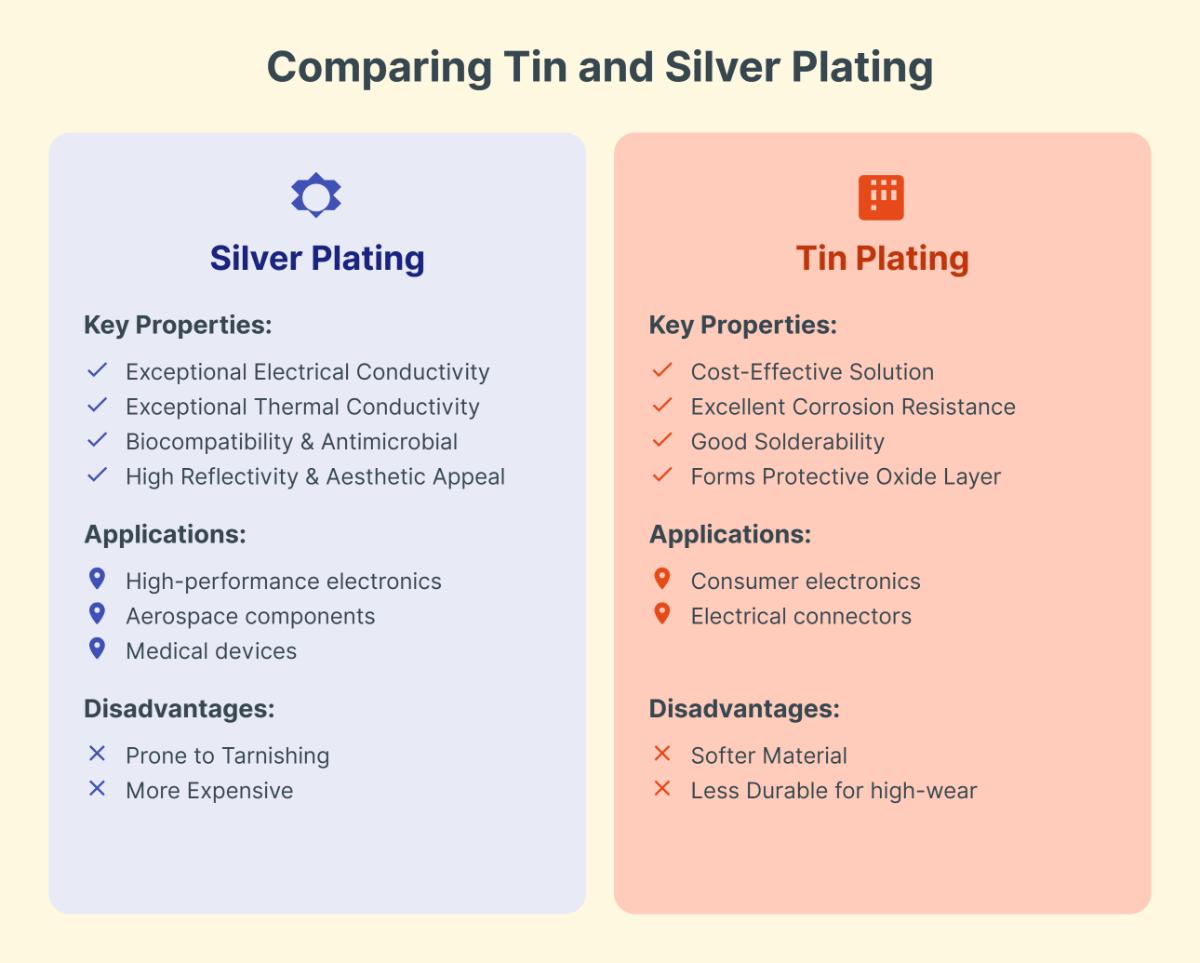
Tin plating and silver plating serve distinct purposes based on their properties and applications. Silver plating offers exceptional electrical and thermal conductivity, making it ideal for high-performance electronics, aerospace, and medical devices due to its biocompatibility and antimicrobial properties. Its high reflectivity and aesthetic appeal are additional advantages. However, silver is prone to tarnishing and is more expensive due to its high market value.
In contrast, tin plating is favored for its cost-effectiveness and excellent corrosion resistance, making it suitable for environments where protection against oxidation is crucial. Tin plating is commonly used in consumer electronics and connectors due to its good solderability and protective oxide layer. However, tin is softer and less durable than silver, limiting its use in high-wear applications.
Corrosion resistance refers to a material’s ability to withstand damage from chemical reactions with its environment. Tin plating generally offers better corrosion resistance than silver plating. Tin forms a protective oxide layer, making it suitable for moist or corrosive environments. In contrast, silver is less resistant, especially in humid conditions or when exposed to sulfur compounds, which can cause tarnishing. However, environmental conditions and base – metal protection also play a role, and silver may be more durable in certain specific scenarios.
When considering which plating is better for soldering, tin plating generally holds the advantage due to its excellent solderability. Tin forms a strong bond with copper, making it ideal for durable connections in electronic components. Its low melting point enhances thermal shock resistance, allowing it to handle sudden temperature changes without compromising the connection integrity. However, tin can oxidize over time, requiring some additional preparation before soldering.
On the other hand, silver plating also offers good solderability and benefits from the fact that silver oxide remains conductive, minimizing the impact of oxidation on soldering. Despite silver’s superior electrical conductivity, its higher cost and tendency to tarnish can make it less practical for general soldering applications unless high-performance requirements justify the expense.
Tin plating and silver plating both have distinct environmental impacts that are essential to consider for sustainable practices. Tin plating involves the use of chemicals such as acids and alkalis, which can generate hazardous waste and pose risks to water sources if not properly managed. The process is also energy-intensive, contributing to greenhouse gas emissions. The extraction of tin from ore can release harmful elements like arsenic and lead, though innovations like ore sorting technology help mitigate these risks.
Silver plating, while more expensive due to the high cost of silver, does not inherently produce more environmental waste than tin plating. However, it shares similar challenges in waste management and chemical handling, requiring careful management to avoid environmental and health hazards. Silver’s superior conductivity can reduce energy consumption in high-performance applications, potentially offsetting some environmental impacts.
Both processes benefit from mitigation strategies such as closed-loop recycling, advanced wastewater treatment, and the adoption of green chemistry practices. These strategies help minimize the ecological footprint, making the processes more sustainable.
When comparing the costs of tin and silver plating, tin plating is generally more cost-effective due to several factors. Firstly, tin is significantly cheaper than silver, resulting in lower raw material costs. Additionally, the tin plating process is simpler and more widely available, which further reduces expenses. This makes tin plating ideal for cost-sensitive applications, such as general electronic components and consumer electronics, where good solderability and corrosion resistance are sufficient.
In contrast, silver plating is more expensive because silver has a higher market value. The complexity of the silver plating process, which involves stringent safety measures due to hazardous materials, also adds to the cost. Despite the higher expense, silver plating is chosen for high-performance applications, such as aerospace and high-frequency electronics, where its superior conductivity and aesthetic appeal are crucial.
Tin plating and silver plating serve distinct purposes across various industries due to their unique properties.
Tin plating is commonly utilized in the electrical and electronics industries for its excellent solderability and corrosion resistance, ensuring reliable connections on circuit boards and connectors. In the automotive and aerospace sectors, it protects fasteners, connectors, and terminals from harsh environments. Additionally, tin plating is prevalent in food packaging, where it offers a non-toxic, corrosion-resistant solution for preserving perishable goods. In the realm of electric vehicles and renewable energy, tin plating enhances conductivity and durability in components like connectors and solar equipment.
On the other hand, silver plating is favored in high-performance electronics due to its superior electrical conductivity, making it ideal for high-frequency electronics, semiconductor devices, and communication systems. In the aerospace and automotive industries, silver plating is used on components requiring high durability and conductivity, such as electrical contacts and sensors. Moreover, silver plating is popular for decorative items like jewelry, thanks to its bright, reflective finish.
Thus, while tin plating is more cost-effective and suitable for general industrial applications, silver plating is preferred for high-performance and aesthetic applications where superior conductivity and appearance are crucial.

