Imagine walking into a historic building and admiring the gleaming, golden fixtures that have stood the test of time. Ever wondered what gives those fixtures their unique luster and durability? The answer lies in the fascinating world of brass, an alloy that has been used for centuries due to its remarkable properties. If you’re just starting to explore the types of brass and their compositions, you’re in the right place.
In this article, we’ll delve into the different types of brass, such as Alpha Brass, Alpha-Beta Brass, and Beta Brass, and break down their specific compositions. We’ll explore the role of copper and zinc in creating this versatile alloy and how varying their proportions can significantly alter its properties. Whether you’re curious about the technical aspects or practical applications in plumbing, musical instruments, and decorative items, this guide has you covered.
Ready to uncover the secrets behind brass and its various forms? Let’s dive in and discover how the composition of this alloy makes it a cornerstone in countless industries.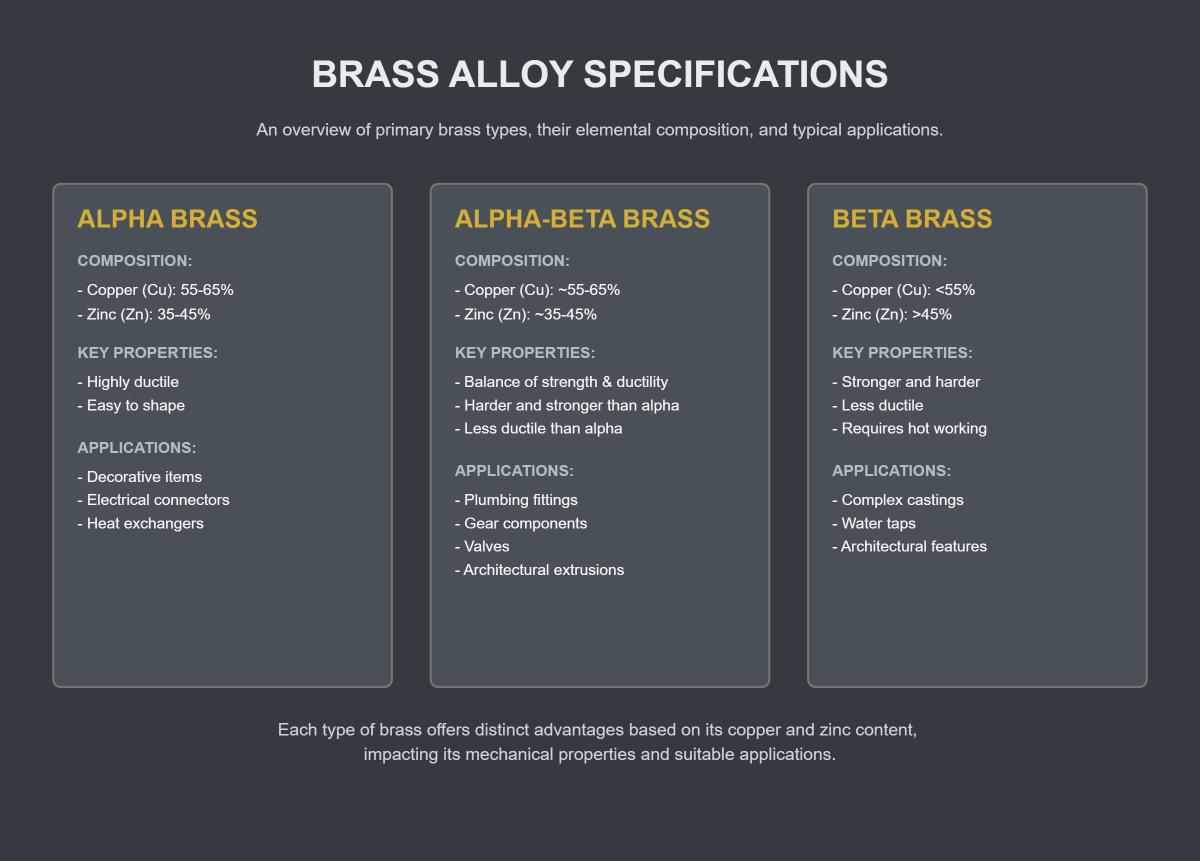
Brass is an alloy primarily composed of copper and zinc. The specific properties of brass can vary significantly depending on the proportions of these two metals, making it a versatile material used in many applications. Typically, the copper content in brass ranges from 55% to 95%, with the rest being zinc. For example, a common ratio is 70% copper and 30% zinc. This type of brass offers a good balance of corrosion resistance, ductility, strength, and hardness, making it suitable for a wide range of uses.
An alloy is a mixture of two or more metals. In the case of brass, copper and zinc are mixed. Copper has excellent thermal and electrical conductivity and resists corrosion well. Zinc makes the alloy stronger and harder and also helps with corrosion resistance.
Think of brass composition as having a larger part of copper and a smaller part of zinc. Copper often has a reddish – brown color, while zinc is lighter and silvery. The relative sizes of these parts change based on the type of brass.
Brass is valuable in numerous industries. It’s widely used in manufacturing musical instruments. Its unique properties give musical instruments a rich and resonant sound. It’s also used for plumbing fixtures. Its corrosion resistance makes it suitable for long – term use in water systems. Decorative items also often use brass because of its appealing appearance and ease of machining.
Brass also has a remarkable feature – it’s antimicrobial, which is super useful in healthcare settings for touch surfaces like door handles and railings. Its ability to resist tarnishing and maintain a bright, shiny appearance further enhances its desirability for decorative purposes. The alloy’s good thermal and electrical conductivity are beneficial in electrical and electronic components, and its corrosion resistance makes it suitable for marine applications.
By exploring the composition and properties of brass, we can appreciate why this alloy has been a material of choice for various applications throughout history. Its adaptability and performance continue to make it an essential material in modern engineering and design.
Alpha brass contains less than 37% zinc, with the remaining composition primarily being copper. This single-phase alloy has a uniform structure throughout, resulting in high ductility and malleability. These properties make alpha brass ideal for creating detailed jewelry pieces or delicate electrical contacts, where intricate forming and bending are essential.
Alpha-beta brass, also known as duplex brass, contains a balanced composition of copper (55% to 65%) and zinc (35% to 45%). This mixture creates a two-phase alloy consisting of both alpha and beta phases. The alpha phase provides ductility, while the beta phase adds strength. This combination makes alpha-beta brass ideal for applications like pipe elbows and tees, which require both strength and formability. Its increased strength compared to alpha brass allows it to withstand higher stress and pressure.
Beta brass contains a higher zinc content, typically ranging from 45% to 50%, with the rest being copper. This composition results in a predominantly beta phase alloy, which is harder and stronger than alpha or alpha-beta brass. Beta brass is less ductile and more difficult to work with, but its high strength makes it suitable for high-stress industrial components like screws, nuts, bolts, and other fasteners that require excellent mechanical properties.
Lead brass includes a small amount of lead (typically around 1% to 3%) added to the copper and zinc alloy. The addition of lead significantly enhances the machinability of the brass, making it easier to cut and shape into precise components. Lead brass is often used in applications requiring detailed machining, such as in the production of faucets, valves, and other plumbing fixtures. However, due to health and environmental concerns associated with lead, the use of lead-free alternatives is becoming more common.
Naval brass contains around 1% tin in addition to copper and zinc. The tin content provides exceptional corrosion resistance, particularly in marine environments. This makes naval brass an excellent choice for applications exposed to seawater and harsh conditions, such as shipbuilding, propellers, and marine hardware. The alloy’s resistance to dezincification ensures long-lasting performance in demanding environments.
Silicon brass includes a small amount of silicon, which enhances the alloy’s strength and corrosion resistance. This type of brass is known for its durability and is commonly used in automotive parts, industrial machinery, and heavy-duty applications. Silicon brass’s ability to maintain its properties under stress and in corrosive environments makes it a reliable material for high-performance components.
Red brass, also known as gunmetal, typically contains about 85% copper, giving it a reddish hue. This high copper content provides excellent corrosion resistance and a pleasing aesthetic appearance. Red brass is often used for outdoor sculptures in public parks, where its reddish hue adds an eye-catching element. Its durability and resistance to tarnishing make it a preferred choice for decorative and functional applications.
Copper is the primary component of brass, contributing significantly to its color, malleability, and resistance to corrosion. The presence of copper gives brass its characteristic yellowish hue, which can range from a reddish tint to a more golden appearance depending on the proportion of zinc. Copper also enhances the material’s workability, making it easier to shape and form into various products.
Zinc is the second key element in brass, and its content varies depending on the type of brass. Zinc greatly increases the strength and hardness of the alloy. Higher zinc content typically results in a stronger and harder brass, which is useful for applications requiring durable and wear – resistant materials, such as screws and bolts. Zinc also contributes to the corrosion resistance of brass, making it suitable for use in environments where the material might be exposed to moisture or other corrosive elements.
Brass alloys must meet quality standards set by organizations like the American Society for Testing and Materials (ASTM) and the European Committee for Standardization (CEN). Following these standards is essential for the reliability and safety of brass components in both industrial and consumer uses.
The corrosion resistance of brass is one of its most notable properties. The copper content in brass forms a protective oxide layer on the surface, which prevents further corrosion. This makes brass an excellent choice for applications exposed to moisture and corrosive environments, such as plumbing fittings and marine hardware. The addition of elements like tin and aluminum can further enhance the corrosion resistance of specific brass alloys, such as naval brass, making them suitable for even more demanding environments.
Here is a comparison of the properties of Alpha Brass, Alpha – Beta Brass, and Beta Brass:
| Property | Alpha Brass | Alpha – Beta Brass | Beta Brass |
|---|---|---|---|
| Copper Content | 65 – 70% | 55 – 65% | 50 – 55% |
| Zinc Content | 30 – 35% | 35 – 45% | 45 – 50% |
| Ductility | High | Moderate | Low |
| Strength | Moderate | High | Very High |
| Corrosion Resistance | High | Moderate to High | Moderate |
| Workability | Excellent | Good | Fair |
| Common Applications | Decorative items, | Plumbing fittings, | Screws, nuts, |
| electrical connectors | gears, valves | bolts |
Brass is popular for decorative items because of its appealing look and versatility. Its golden hue can be polished to a high shine or given a patina finish for a vintage look. Common decorative applications include:
Brass is widely used in plumbing due to its excellent corrosion resistance and workability. It is suitable for:
The acoustic properties of brass make it a preferred material for various musical instruments. It is used in:
Brass’s low friction and strong mechanical properties make it perfect for gears and bearings, which helps reduce wear and extend machinery lifespan. It is also used in:
Brass conducts electricity well, making it ideal for connectors and terminals in various electrical applications. It is also used in:
Brass is commonly used in the production of ammunition casings. Its properties make it ideal for:
Brass is used in architecture and construction for its durability and decorative appeal. It is chosen for:
Brass’s sustainability and corrosion resistance make it suitable for various industries. It is recyclable and retains its properties after recycling, contributing to environmental conservation. Its resistance to corrosion ensures long-lasting performance in applications exposed to moisture and harsh conditions, such as marine hardware and outdoor fixtures.
Below are answers to some frequently asked questions:
Brass is an alloy made primarily of copper and zinc, and its composition can vary to create different types with unique properties. The main types of brass are:
Each type of brass offers distinct advantages based on its copper and zinc content, impacting its mechanical properties and suitable applications.
Alpha brass, which contains 65-70% copper and 30-35% zinc, is highly ductile and is typically used for decorative items such as jewelry and ornamental pieces, as well as electrical connectors and architectural components like door hardware and fixtures. Alpha-Beta brass, with 55-65% copper and 35-45% zinc, strikes a balance between strength, ductility, and corrosion resistance, making it suitable for plumbing fittings, valves, gears, mechanical parts, and general hardware components like locks and hinges. Beta brass, containing 50-55% copper and 45-50% zinc, is stronger and harder, ideal for machine parts, valves, gears, and fasteners that require high strength. Each type of brass is chosen based on the specific needs of the application, including considerations for strength, durability, and ease of forming.
The composition of brass, mainly the ratio of copper and zinc and the presence of additives, greatly affects its properties. Copper provides malleability, ductility, and excellent thermal and electrical conductivity. Zinc increases strength and corrosion resistance. Alpha brasses, with more copper, are highly ductile, while beta brasses, with more zinc, are stronger but less ductile. Additives like lead improve machinability, and arsenic enhances resistance to dezincification. The copper – to – zinc ratio also influences color, ranging from bright to pale yellow. Additionally, brass has antibacterial properties thanks to its copper content.
Copper and zinc are used to make brass because they combine to create an alloy with beneficial properties suitable for a wide range of applications. Copper contributes to brass’s malleability, ductility, and corrosion resistance, making it easy to shape and form. It also gives brass its distinctive golden color and enhances electrical and thermal conductivity. Zinc, on the other hand, increases the strength and durability of the alloy, providing resistance to wear and impact. The zinc content also affects the color of brass, with higher percentages leading to a whiter appearance and lower percentages producing a redder hue. Together, these metals create a versatile and functional material used in various industries, from decorative items to plumbing fittings and musical instruments.
To identify different types of brass, you can use several methods. First, visually inspect the brass. It usually has a bright, golden – yellow appearance, but the color can range from yellow to reddish based on the copper – to – zinc ratio. A magnetic test is also simple: brass is non – magnetic. For a scratch test, if it’s solid brass, it will reveal a shiny, golden – yellow color. You can do a weight test as well; brass is dense and heavy. When you strike brass, it produces a clear, high – pitched sound. Lastly, an acid test can be used to verify the composition by observing its reaction with dilute acid.
Brass, an alloy of copper and zinc, offers several benefits over other metals. It is more corrosion-resistant than stainless steel and lasts longer, making it great for plumbing. Compared to copper, brass has better structural integrity due to zinc. It is highly malleable and ductile, easier to shape than stainless steel, suitable for decorative items and machinery parts. Brass conducts electricity better than stainless steel, though not as well as pure copper. It has antimicrobial properties because of copper, useful in healthcare and water filtration. Its gold – like appearance is appealing for decoration. Also, it’s cost – effective compared to bronze or gold, making it a versatile and popular choice.

