Imagine a world without the strength and durability of steel – skyscrapers would crumble, bridges would collapse, and our modern infrastructure would be but a distant dream. One of the unsung heroes in the realm of metallurgy that ensures the robustness of steel is manganese. This often-overlooked element plays a pivotal role in enhancing the mechanical properties of steel, making it tougher, more durable, and resistant to wear and tear. But how exactly does manganese achieve this, and what implications does it have for the steel industry? In this technical deep dive, we will explore the critical contributions of manganese to steel production, its impact on hardness and toughness, and the myriad applications that benefit from this alloy. Join us as we unravel the complexities of manganese steel, uncovering its benefits, limitations, and the fascinating science behind its transformative power. Ready to delve into the world of manganese and its steel-enhancing magic? Let’s get started.
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal found in various minerals and widely distributed in the earth’s crust. Manganese does not occur naturally as a free element; instead, it is typically combined with iron and other minerals. It is an essential trace element for all known living organisms, playing a crucial role in various biological processes.
In the field of metallurgy, manganese is indispensable due to its significant impact on the properties of steel. It is the second most common alloying element used in steel production, after carbon. In steelmaking, manganese primarily serves to:
The alloying process involves introducing manganese into molten steel during steelmaking. This can be done using various manganese-containing materials such as ferromanganese, silicomanganese, or manganese ore. The key steps in the alloying process include:
The American Society for Testing and Materials (ASTM) sets standards for the composition and mechanical properties of manganese steel. Compliance with ASTM standards ensures that the steel meets specific requirements for various applications. Key ASTM standards related to manganese steel include:
Meeting these standards guarantees that manganese steel products are reliable, durable, and fit for their intended uses.
Adding manganese to steel increases its hardness. This is due to the formation of manganese carbides, which are hard particles that enhance the overall hardness of the steel matrix. Manganese also contributes to the hardenability of steel, allowing it to be heat-treated to higher hardness levels.
In the mining industry, manganese steel is used in rock crushers and other heavy-duty equipment. The high hardness of manganese steel allows these components to withstand the constant abrasion and impact from rocks and other hard materials, significantly extending their service life.
| Manganese Content (%) | Hardness (HB) |
|---|---|
| 0-1 | 120-150 |
| 1-2 | 150-180 |
| 2-3 | 180-210 |
| 3-4 | 210-240 |
| 4-5 | 240-270 |
| 5-6 | 270-300 |
| 6-7 | 300-330 |
| 7-8 | 330-360 |
| 8-9 | 360-390 |
| 9-10 | 390-420 |
| 10-11 | 420-450 |
| 11-12 | 450-480 |
| 12-13 | 480-510 |
| 13-14 | 510-540 |
Manganese enhances the ability of steel to harden through heat treatment, improving both its toughness and hardenability. This results in a more uniform and tougher steel.

Manganese improves steel’s corrosion resistance by acting as a deoxidizer and removing impurities. This ensures a cleaner microstructure, which is less susceptible to corrosion. Manganese steel is particularly effective in environments where both wear resistance and corrosion resistance are required.
In marine applications, manganese steel is used for components exposed to seawater and abrasive conditions. The combination of wear resistance and corrosion resistance ensures long-term performance and durability.
Manganese boosts steel’s yield and tensile strength, enabling it to endure heavy loads and impacts. This makes manganese steel ideal for high-stress applications.
One of the key challenges in metallurgy is balancing strength and ductility. Manganese steel manages to achieve high strength without significant loss of ductility. This balance is essential for applications that require both toughness and the ability to deform without breaking.
| Manganese Content (%) | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| 0-1 | 250-300 | 400-450 | 25-30 |
| 1-2 | 300-350 | 450-500 | 22-27 |
| 2-3 | 350-400 | 500-550 | 20-25 |
| 3-4 | 400-450 | 550-600 | 18-23 |
| 4-5 | 450-500 | 600-650 | 16-21 |
| 5-6 | 500-550 | 650-700 | 15-20 |
| 6-7 | 550-600 | 700-750 | 14-19 |
| 7-8 | 600-650 | 750-800 | 13-18 |
| 8-9 | 650-700 | 800-850 | 12-17 |
| 9-10 | 700-750 | 850-900 | 11-16 |
| 10-11 | 750-800 | 900-950 | 10-15 |
| 11-12 | 800-850 | 950-1000 | 9-14 |
| 12-13 | 850-900 | 1000-1050 | 8-13 |
| 13-14 | 900-950 | 1050-1100 | 7-12 |
In the construction industry, manganese steel is used in excavator buckets and loader teeth. These components are subjected to constant wear and impact from materials like gravel and concrete. The high wear resistance of manganese steel ensures that these parts last longer, reducing downtime and maintenance costs.
Railroad components, such as switches and crossings, benefit from the impact resistance of manganese steel. The ability to absorb and withstand repeated impacts without significant wear or deformation ensures the reliability and safety of railway operations.
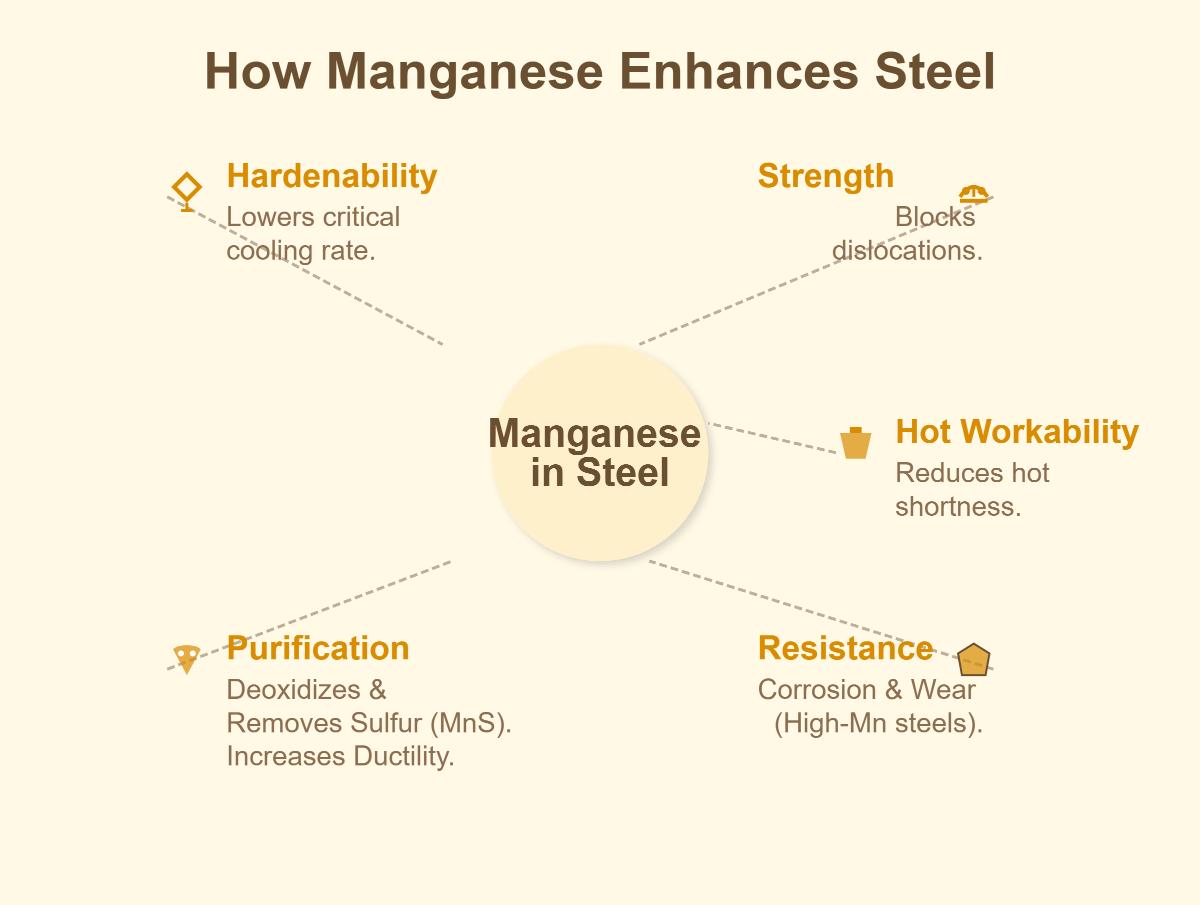
Manganese significantly improves the hardenability of steel, which is its ability to harden deeply and uniformly during heat treatment. This enhancement is achieved by lowering the critical cooling rate, allowing the steel to harden more effectively and evenly. As a result, steels with higher manganese content exhibit increased hardness and yield strength. Recent studies have demonstrated that increasing manganese content can substantially boost tensile strength without compromising elongation, resulting in a better strength-toughness balance.
Manganese enhances hot workability by reacting with sulfur to form manganese sulfide, preventing hot shortness and facilitating easier shaping and machining of steel. This reaction ensures that steel can be processed at high temperatures without becoming brittle, leading to the production of high-quality components.
Manganese plays a vital role as a deoxidizer in steel production. It reacts with dissolved oxygen in molten steel, forming manganese oxides that can be easily removed. This process is crucial for producing steel with fewer impurities, leading to improved mechanical properties and durability. Cleaner steel results in enhanced performance and longevity, making manganese an essential element in high-performance steel.
High-manganese steels exhibit enhanced corrosion resistance, making them ideal for use in environments where exposure to corrosive elements is a concern, such as marine applications. Manganese improves steel’s microstructure, reducing impurities that can initiate corrosion. This ensures that manganese steel components maintain their integrity and performance over extended periods in harsh conditions.
Manganese steel, particularly Hadfield steel with high manganese content (11-14%), is renowned for its exceptional impact resistance. This type of steel work-hardens under impact, meaning its surface hardness increases significantly without becoming brittle. This property makes manganese steel highly suitable for applications involving heavy impacts, such as railway track components, rock crushers, and other heavy-duty equipment.
Manganese steel is well-known for its impact resistance but is less effective against abrasion from fine particles like sand. In such applications, manganese steel wears similarly to mild steel and is outperformed by heat-treated high-carbon steels. However, for applications where impact resistance is paramount, manganese steel remains the preferred choice.
Manganese steel, especially in high-carbon and high-manganese compositions, exhibits remarkable mechanical properties, including high tensile strength and elongation. These properties make it suitable for demanding applications that require materials to withstand significant stress and deformation. The ability to balance strength and ductility is one of the critical advantages of manganese steel, enabling its use in diverse and challenging environments.
The beneficial impacts of manganese on steel properties are evident in various specific applications. In mining equipment, the enhanced hardness and impact resistance of manganese steel ensure the durability and longevity of components such as rock crusher jaws and conveyor parts. In the construction industry, manganese steel is used in excavator buckets and loader teeth, providing excellent wear resistance and reducing maintenance costs. Additionally, in railroad applications, manganese steel improves the performance and reliability of track components subjected to continuous heavy impacts.
Manganese steel is widely used in traditional industries because of its exceptional mechanical properties, such as high impact strength, wear resistance, and the ability to harden under stress.
In the mining industry, manganese steel is invaluable for its durability and resistance to wear, commonly used in rock crushers, excavator buckets, and grinding equipment. The ability of manganese steel to withstand harsh conditions and intense wear makes it ideal for these applications. For example, rock crusher jaws and cone crushers benefit from the high hardness and toughness, ensuring longer service life and reduced downtime.
Manganese steel is also prominent in construction machinery, where components like cement mixer liners and concrete plant wear parts need to withstand abrasion and impact. The material’s robustness ensures that these parts remain functional over extended periods, reducing maintenance costs and enhancing operational efficiency. Excavator buckets and loader teeth are prime examples where manganese steel’s wear resistance is crucial.
The railroad industry relies heavily on manganese steel for critical components like tracks, switches, and crossings. The high impact resistance and durability of manganese steel ensure reliable performance under the stresses of heavy rail traffic. These characteristics help prevent deformation and wear, contributing to safer and more efficient railway operations.
In the renewable energy sector, manganese steel is used in wind turbine components. The material’s ability to withstand high stress and impact loads is essential for the longevity and reliability of these structures. The wind turbine blades and tower components benefit from the strength and toughness of manganese steel, ensuring they can endure the dynamic forces experienced during operation.
Advanced manufacturing processes are increasingly using manganese steel for precision components that require durability and wear resistance, such as high-precision tools and dies. The material’s ability to maintain structural integrity under repeated stress makes it suitable for manufacturing complex parts. These applications benefit from the steel’s work-hardening properties, which enhance surface hardness and extend the life of the components.
Manganese steel’s toughness and non-magnetic properties make it ideal for security and safety applications, including prison windows, safes, and fireproof cabinets. The material’s resistance to cutting and impact ensures that these security products provide reliable protection against tampering and forced entry.
In agriculture, manganese steel is employed in plough shares and disc harrows. These components are subjected to significant wear and tear during soil cultivation. The high durability and wear resistance of manganese steel improve the lifespan of agricultural equipment, reducing the frequency of replacements and maintenance.
One of the key properties of manganese steel is its ability to work-harden. When subjected to impact or abrasion, the surface hardness of the steel increases, making it even more resistant to wear. This property is particularly beneficial in applications like crushers and wear plates, where components experience constant heavy impacts.
Manganese steel offers a unique balance of high strength and ductility, allowing it to absorb and distribute impact forces effectively. This makes it suitable for applications requiring both toughness and the ability to deform without breaking, such as railroad tracks and heavy-duty construction equipment.
While manganese steel provides good protection against corrosion, it is especially effective in environments where both wear resistance and corrosion resistance are required. For instance, marine applications benefit from manganese steel’s ability to withstand seawater exposure and abrasive conditions.
Manganese boosts the mechanical properties of steel, increasing its strength and durability. The primary benefits include:
Manganese acts as a deoxidizer, removing impurities from steel and improving its durability and strength. By binding with sulfur and oxygen, manganese minimizes the formation of iron sulfide, which can lead to cracking and brittleness.
Although manganese steel has a higher initial cost due to complex alloying, its long service life and low maintenance can save money over time. Industries that use manganese steel can benefit from lower downtime and fewer replacements, making it a cost-effective choice in the long run.
Manganese steel contributes to sustainability by providing a longer service life for components, reducing the need for frequent replacements and the associated environmental impact of manufacturing new parts. Additionally, the recycling of manganese steel helps minimize waste and supports sustainable practices in the metal industry.
While manganese steel offers better corrosion resistance than carbon steel, it does not match the performance of stainless steel. In highly acidic or alkaline environments, manganese steel can degrade significantly, limiting its use in certain applications.
Manganese steel becomes brittle at low temperatures, increasing the risk of fractures. This limitation necessitates careful handling and processing, such as preheating and avoiding sudden temperature changes, to mitigate the risk of brittleness.
The high melting point of manganese steel makes it harder to manufacture, leading to more energy use and longer processing times. Additionally, the alloying process is complex and requires precise control, contributing to higher production costs.
Manganese steel is not ideal for applications involving fine abrasive materials like sand. In such conditions, it wears similarly to mild steel. For tasks that involve fine particle abrasion, alternative materials like heat-treated high-carbon steels are often preferred.
Manganese steel is widely used in industries requiring high strength and durability. Key applications include:
Manganese steel, also known as Hadfield steel or mangalloy, is a distinctive steel alloy primarily composed of 11-14% manganese and 0.8-1.4% carbon, with iron making up the rest. This high manganese content imparts unique properties to the steel, making it highly valued in various industrial applications, particularly those involving high impact and wear.
Manganese steel is known for its remarkable ability to absorb substantial energy without breaking, making it perfect for high-impact applications like rock crushers and railroad tracks.
One of the standout features of manganese steel is its wear resistance. When subjected to impact or abrasive conditions, the steel forms a hard surface layer while maintaining a tough inner core. This self-hardening feature, called work-hardening, significantly extends the lifespan of components used in abrasive conditions.
Work-hardening is a process where the steel becomes harder and more wear-resistant with continuous use. This property is particularly beneficial for parts that undergo constant impact, as the surface hardens without becoming brittle, ensuring durability.
Stainless steel, which contains at least 10.5% chromium, offers much better corrosion resistance than manganese steel, making it ideal for marine and chemical processing industries.
While stainless steel excels in corrosive environments, manganese steel is better suited for high-impact and high-wear applications. For example, manganese steel is preferred in mining and construction industries, where the material’s ability to withstand severe wear and impact is crucial.
High-manganese steels can reach tensile strengths of up to 1000-1200 MPa, providing exceptional strength and toughness essential for durable applications. This improvement in strength and toughness is critical for applications requiring durable and resilient materials.
Manganese also improves the corrosion resistance of steel, particularly in high-manganese alloys. This makes them suitable for use in marine environments and other applications where both wear and corrosion resistance are necessary.
Manganese lowers the critical cooling rate, allowing deeper and more uniform hardening during heat treatment. This property enhances the steel’s hardenability, making it more effective for producing components that require consistent hardness throughout.
Manganese steel is commonly used in industrial applications such as railway tracks, construction materials, heavy machinery, and mining equipment. Its strength and wear resistance make it ideal for these demanding environments.
Innovative uses of manganese steel are emerging, including its exploration in samurai sword crafting. The material’s impact resistance and durability make it a promising candidate for creating high-performance blades.
Manganese steel is notoriously difficult to machine due to its work-hardening properties. This necessitates the use of special tools and techniques to effectively cut and shape the material.
Welding manganese steel requires precise temperature control; improper techniques can cause the welded joints to become brittle, which can compromise their strength.
As technology advances, the unique properties of manganese steel are expected to find applications in various industries, from traditional construction to innovative fields like modern sword crafting. Addressing the challenges of machining and welding will be crucial for expanding the use of manganese steel in new and existing applications.
Below are answers to some frequently asked questions:
Manganese significantly enhances steel properties. It improves hardenability by lowering the critical cooling rate for a uniform hardening effect. It also contributes to strength by forming compounds that block dislocations. Manganese reduces hot shortness, enhancing hot workability, and acts as a deoxidizer, reacting with oxygen in molten steel. It removes sulfur by forming manganese sulfide, increasing ductility. High – manganese steels show better corrosion and wear resistance, making them suitable for marine and high – impact applications.
Manganese steel, a blend of manganese and steel, is well – known for its toughness, wear resistance, and work hardening. In traditional industries, it’s used in mining for rock crushers and drilling components, in construction for bulldozer blades and excavator buckets, in railroads for crossings and switch points, and in the cement industry for mixer and crusher parts. In emerging industries, while manganese compounds are vital for lithium – ion batteries, the non – magnetic manganese steel is suitable near electrical components. It’s also used in security and defense for safes and anti – drill plates.
Manganese is a vital alloying element in steel production, providing several benefits while also presenting some limitations, especially concerning ASTM standards.
Benefits of using manganese in steel include improved strength and hardness, enhanced wear resistance, and unique work-hardening properties. Manganese increases the hardenability of steel, making it suitable for applications requiring high durability. It also contributes to better corrosion resistance compared to many other steel types, though it still requires maintenance in corrosive environments.
However, the use of manganese in steel comes with limitations. High manganese content can increase brittleness, particularly at low temperatures, and the material is challenging to machine due to its hardness and work-hardening characteristics. Additionally, while manganese steel has better corrosion resistance than many steels, it is still susceptible to corrosion and requires regular maintenance. The thermal properties of manganese steel, such as higher thermal expansion and lower thermal conductivity, can lead to internal stresses and cracking during temperature fluctuations.
ASTM standards ensure that steel materials meet specific criteria for properties, testing methods, and applications. Manganese steel often meets or exceeds these standards, particularly in terms of wear resistance and toughness, making it ideal for harsh environments. However, users must verify that the specific manganese steel alloy complies with relevant ASTM specifications for their intended applications.
Manganese improves the hardness of steel by enhancing its hardenability, which is the ability of steel to be hardened through heat treatment. Manganese lowers the critical cooling rate, allowing the hardening effect to penetrate more uniformly and deeply into the steel, creating a tougher and more stable microstructure. This is achieved by manganese’s role in preventing the formation of unwanted compounds like ferrite and cementite, and promoting the formation of fine ferrite and pearlite, which contribute to increased hardness and strength.
A real-world example of this is Hadfield Manganese Steel, also known as Mangalloy, which contains 11% to 14% manganese. Initially soft, this steel becomes significantly harder under repeated impacts, with surface hardness reaching up to 550 Brinell Hardness Number (BHN). This property makes it ideal for high-impact applications such as railway tracks, mining equipment, and construction machinery.
Industries that benefit the most from manganese steel include construction and heavy machinery, mining and quarrying, the railroad industry, security and defense, and manufacturing and metal recycling. Manganese steel’s exceptional mechanical properties—such as high strength, hardness, and resistance to wear and impact—make it indispensable in these sectors.
In construction and heavy machinery, manganese steel is used in components like excavator buckets and crusher jaws, where its durability reduces maintenance costs and downtime. In mining and quarrying, its wear resistance extends the service life of equipment such as jaw-crusher plates and impact hammers. The railroad industry utilizes manganese steel for track components, ensuring the integrity and safety of railway operations under constant stress. In security and defense, its resistance to cutting tools makes it ideal for high-security applications like safes and prison window frames. Lastly, in manufacturing and metal recycling, manganese steel’s durability and impact resistance are essential for processing heavy-duty materials and creating high-quality tools.
Tables and diagrams play a crucial role in understanding the properties of manganese steel by providing visual and comparative data that highlight the impact of manganese content on various mechanical properties. For instance, tables can succinctly display the relationship between different manganese concentrations and corresponding changes in yield strength, tensile strength, and hardness. This comparative approach allows for a clear understanding of how increasing manganese content enhances hardness and wear resistance while potentially affecting ductility.
Diagrams, such as phase diagrams, are essential for visualizing how manganese influences the structural phases of steel at different temperatures and compositions. These diagrams help in understanding the austenite stability and the transformation processes that contribute to the material’s mechanical properties, such as work hardening. Additionally, diagrams illustrating hardenability curves and corrosion resistance can elucidate the benefits of manganese steel in specific applications, such as mining equipment and railroad components.
By incorporating these visual aids, readers can better grasp the complex interactions between manganese and steel, facilitating a more comprehensive understanding of the alloy’s performance characteristics and its suitability for various industrial applications.

