Picture a material that radiates a rich golden hue, yet can shift to a soft pink or a muted silver with just a change in its composition. This fascinating chameleon is brass, an alloy that has captivated artisans, engineers, and designers for centuries. But what exactly influences the color of brass, and what makes it so versatile?
In this article, we delve into the intricate world of brass, exploring the pivotal role that copper and zinc play in determining its color. You will learn how varying these elements can result in the vibrant spectrum seen in red, yellow, and silver brass. Beyond its aesthetic appeal, brass boasts a remarkable set of properties, including impressive durability, tensile strength, and corrosion resistance, making it a preferred choice in a myriad of applications from architecture to marine engineering.
Join us as we uncover the secrets behind brass’s unique characteristics and discover how this timeless alloy continues to shape industries and design trends. Ready to explore the science and artistry behind brass? Let’s dive in.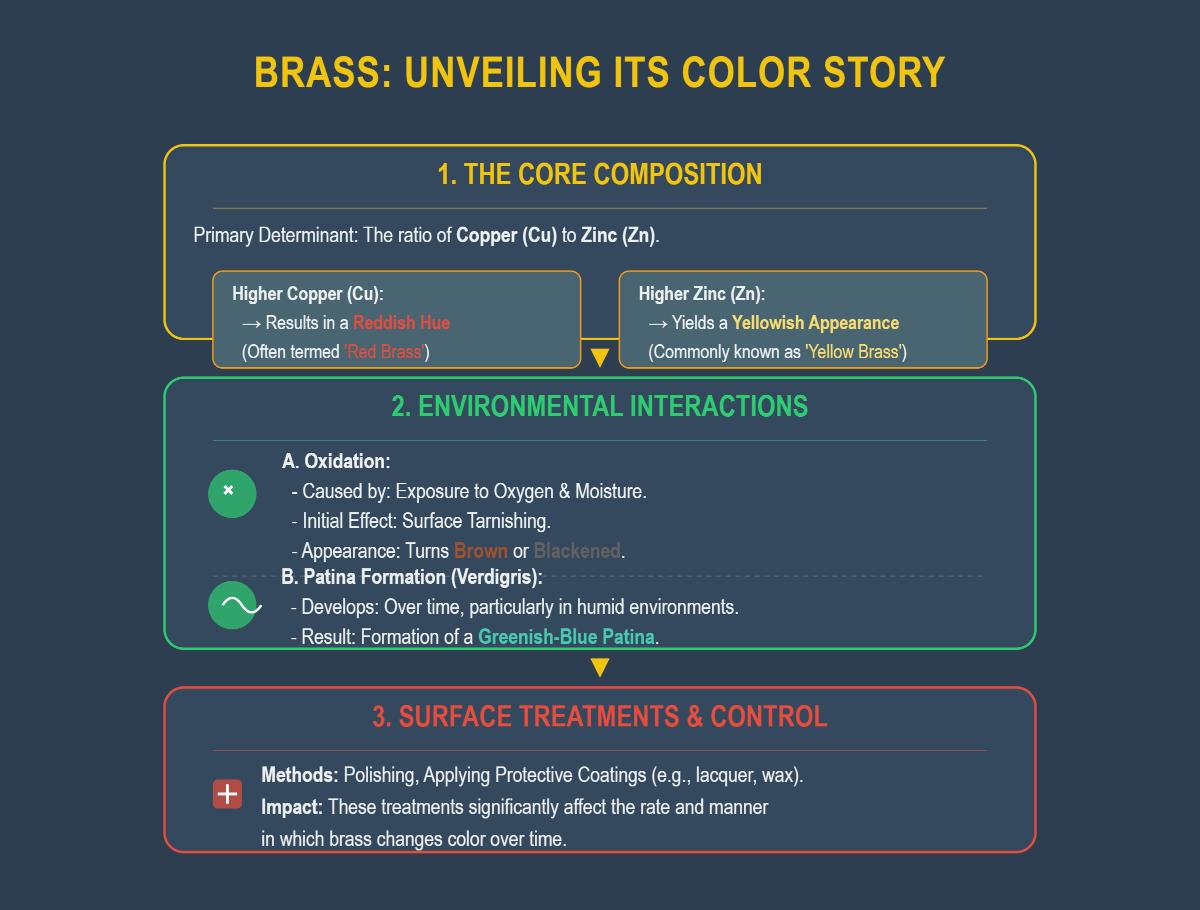
Brass is an alloy primarily composed of copper and zinc. The specific proportions of these two elements can vary, leading to different types of brass with distinct properties and applications. Brass is widely appreciated for its excellent machinability, corrosion resistance, and attractive appearance.
Copper and zinc are the main components of brass, each adding distinct properties to the alloy. Copper is known for its ductility, thermal and electrical conductivity, and resistance to corrosion. Zinc, on the other hand, enhances the strength and hardness of the alloy while maintaining good workability.
The color of brass is significantly influenced by its composition, particularly the ratio of copper to zinc. As the zinc content increases, the color of the brass tends to shift from a reddish or golden hue to a more yellowish tone. This shift is not only aesthetic but also indicative of changes in the alloy’s mechanical properties.
Copper content plays a crucial role in determining the color and properties of brass. Higher copper content, such as in an 85/15 brass (85% copper, 15% zinc), imparts a reddish or golden color to the alloy. This type of brass is often used in applications where a visually appealing finish is desired, such as in decorative arts and jewelry.
Zinc content also significantly impacts the color and characteristics of brass. As the zinc proportion increases, the alloy has a more muted yellow color and is often used in industrial applications that require high durability. For instance, a 60/40 brass (60% copper, 40% zinc) exhibits these properties.
Brass alloys may include small amounts of other elements like lead (for machinability), aluminum (for corrosion resistance), tin (for strength and corrosion resistance), and manganese (for strength and wear resistance). These alloying elements allow for the customization of brass properties to meet the needs of various applications.
Brass color varies based on its composition, with common variations including:
Red brass, with about 85% copper and 15% zinc, has a reddish or golden hue, making it ideal for decorative items, plumbing, and jewelry. Its high copper content also provides excellent corrosion resistance.
Yellow brass, often composed of 70% copper and 30% zinc, displays a classic golden-yellow color. This type of brass is widely used in musical instruments, hardware, and general-purpose applications due to its balanced properties and appealing appearance.
Silver brass, also known as nickel silver or German silver, contains copper, zinc, and nickel. The addition of nickel gives this alloy a silvery appearance and enhances its strength and corrosion resistance. Silver brass is commonly used in cutlery, musical instruments, and decorative items.
Brass colors can be represented using RGB and Hex codes, which are useful for digital design and manufacturing purposes. Some common codes include:
These color codes help standardize the appearance of brass in various industries, ensuring consistency and accuracy in production and design.
Visual representation of brass colors can aid in selecting the right type of brass for specific applications. Comparing the colors side by side can highlight the differences in hue and help in making informed decisions based on aesthetic and functional requirements.
Brass has a variety of mechanical properties, making it a versatile material for many uses. Its mechanical characteristics are primarily influenced by its composition, particularly the copper-to-zinc ratio.
Tensile strength measures how much tension brass can handle before breaking. The tensile strength of brass can vary significantly depending on its composition. Alpha brasses, which contain less than 37% zinc, typically have lower tensile strength but higher ductility, making them suitable for applications requiring extensive forming. In contrast, alpha-beta brasses, with higher zinc content (37-45%), exhibit greater tensile strength and hardness, making them ideal for applications requiring durability and wear resistance.
Brass is known for its excellent malleability and ductility, allowing it to be easily formed into various shapes without cracking. These properties make brass a preferred material for intricate components, such as musical instruments, decorative items, and fittings. Alpha brasses can be cold worked extensively without losing strength, whereas alpha-beta brasses are better for hot working.
Durability is a significant attribute of brass, contributing to its widespread use in demanding environments. Brass alloys, especially those with higher zinc content, offer increased hardness and wear resistance. This makes them suitable for applications such as gears, bearings, and valves, where long-term performance and resistance to mechanical stress are essential.
Brass is highly valued for its corrosion resistance, particularly in environments where exposure to moisture and chemicals is common. The inherent corrosion resistance of brass is due to the formation of a protective oxide layer on its surface, which prevents further degradation. This property is crucial for applications in marine, plumbing, and outdoor environments.
The corrosion resistance of brass, which forms a protective oxide layer, can be further improved by adding elements like tin, aluminum, or nickel. These additions can enhance the stability of the protective layer and reduce the rate of corrosion. For instance, naval brass, which includes a small percentage of tin, is specifically designed for marine applications where resistance to seawater corrosion is paramount.
Brass possesses excellent thermal conductivity, making it an ideal material for heat exchangers, radiators, and other applications where efficient heat transfer is required. The high thermal conductivity of brass is primarily due to its copper content, which is known for its ability to conduct heat effectively.
While not as conductive as pure copper, brass still offers good electrical conductivity, making it suitable for electrical connectors, terminals, and other components where reliable electrical performance is necessary. The balance of conductivity and mechanical strength in brass makes it a practical choice for many electrical applications.
Brass has natural antibacterial properties, which inhibit the growth of bacteria on its surface. This is especially useful in healthcare settings, for items like medical instruments, hospital fixtures, and frequently touched surfaces such as door handles and railings. The antibacterial nature of brass helps maintain hygiene and reduce the spread of infections.
| Property | Description |
|---|---|
| Tensile Strength | Varies with composition, higher zinc = higher strength |
| Malleability/Ductility | Excellent, especially in alpha brasses |
| Durability | High, with increased hardness in higher zinc alloys |
| Corrosion Resistance | Naturally high, can be enhanced with alloying elements |
| Thermal Conductivity | Excellent, ideal for heat transfer applications |
| Electrical Conductivity | Good, suitable for electrical components |
| Antibacterial Properties | Inhibits bacterial growth, ideal for sanitary applications |
Understanding these key properties of brass allows for its effective application across various industries, ensuring optimal performance and longevity in both functional and decorative uses.
Brass alloys differ mainly in the copper-to-zinc ratio, and sometimes include other elements to improve specific qualities. Here are some common types of brass:
Red brass, which contains 85% copper and 15% zinc, is valued for its reddish hue, excellent corrosion resistance, and is commonly used in plumbing, marine applications, and decorative arts.
Yellow brass, with about 60-70% copper and 30-40% zinc, features a bright yellow color. It is widely used in applications such as musical instruments, architectural fittings, and hardware due to its balanced mechanical properties and attractive appearance.
Silver brass, also known as nickel silver or German silver, includes nickel in its composition, which gives it a silvery appearance. This alloy is used in decorative items, cutlery, and musical instruments because of its enhanced strength, corrosion resistance, and appealing look.
Brass is utilized across various industries due to its unique properties. Here are some key applications:
In industries, brass is preferred for its ease of machining, strength, and resistance to corrosion. It’s used in components like gears, bearings, valves, and fittings. Cartridge brass (Alloy 260), known for its excellent cold-working properties, is often employed in the production of ammunition and automotive parts.
Brass’s aesthetic appeal makes it a popular choice for decorative applications. Items such as jewelry, architectural fittings, and musical instruments often utilize brass for its attractive finish and ability to be shaped into intricate designs. Yellow brass and silver brass are particularly favored in these applications due to their visual appeal.
Naval brass (Alloy 464), which contains a small amount of tin, is specifically designed for marine environments. Its high corrosion resistance makes it ideal for use in shipbuilding, seawater piping, and other marine hardware. Red brass is also commonly used in plumbing and marine settings due to its durability and resistance to corrosion.
In architecture, brass is used for its aesthetic qualities and durability. It is employed in fixtures, railings, and door handles. In plumbing, brass’s corrosion resistance and workability make it an excellent material for pipes, fittings, and valves. Its ability to resist dezincification, a form of corrosion, ensures long-lasting performance in water systems.
Knowing the different types of brass and their uses helps in choosing the right alloy for each application, ensuring the best performance and durability.
Brass is naturally resistant to corrosion, but its resistance can be further improved using various techniques.
Incorporating elements such as aluminum, tin, and nickel into brass can significantly enhance its corrosion resistance. Adding aluminum forms a protective aluminum oxide layer, which is particularly beneficial in marine environments. Tin enhances resistance to seawater and dezincification, making it ideal for naval brass used in marine hardware. Nickel strengthens the alloy and improves its resistance to tarnishing and general corrosion.
Applying coatings to brass can provide extra protection against corrosion.
The durability of brass can be improved through heat treatment and mechanical processing, which alter the microstructure and mechanical properties of the alloy.
Heat treatment processes like annealing and quenching can optimize brass’s mechanical properties.
Mechanical processing techniques such as cold working and hot working can enhance the strength and hardness of brass.
Improving brass properties can be achieved through systematic approaches. Here are some step-by-step guides for common enhancement methods:
By following these methods, the properties of brass can be tailored to meet specific application requirements, ensuring optimal performance and longevity.
Brass is essential in construction due to its strength and resistance to environmental conditions. Common applications include:
In the engineering sector, brass is valued for its versatility and performance under stress. Applications include:
Brass’s malleability and attractive finish make it ideal for creating intricate jewelry and elegant home decor items like candlesticks, vases, and picture frames.
Brass has seen a resurgence in popularity in modern design due to its timeless appeal and versatility. Current trends include:
Brass is a sustainable choice in material selection due to its recyclability. It can be melted down and reused without losing its properties, making it an environmentally friendly option for industries focused on sustainability.
Designing with brass requires understanding its properties and maintenance needs. Some expert tips include:
By leveraging brass’s unique properties and following best practices in design and maintenance, industries can enhance the performance and longevity of brass components in various applications.
Below are answers to some frequently asked questions:
The color of brass is primarily influenced by its composition, specifically the ratio of copper to zinc. Brass is an alloy of these two metals, and variations in their proportions can lead to different colorations. Higher copper content results in a reddish hue, often referred to as “red brass,” while higher zinc content yields a yellowish appearance, known as “yellow brass.” Additionally, environmental factors such as oxidation and patina formation play significant roles. When exposed to oxygen and moisture, brass initially tarnishes and may develop a brown or blackened appearance. Over time, especially in humid environments, it can form a greenish-blue patina called verdigris. Surface treatments like polishing or applying protective coatings can also affect the rate at which brass changes color.
Brass is an alloy primarily composed of copper and zinc, with the specific proportions of these metals significantly influencing its properties. Key properties of brass include:
These properties make brass a versatile material used across various industries for both functional and decorative purposes.
Brass is an alloy primarily composed of copper and zinc, and its types vary based on their specific compositions and properties, which determine their uses.
Red brass, also known as semi-red brass or gunmetal, contains a higher copper content (typically around 85% copper and 15% zinc). It is valued for its excellent corrosion resistance and is commonly used in plumbing, marine hardware, and for decorative items.
Yellow brass, with a composition of about 60-70% copper and 30-40% zinc, has a bright, gold-like appearance. It is frequently used in applications requiring a combination of good corrosion resistance and aesthetic appeal, such as musical instruments, architectural fittings, and hardware.
Silver brass, also known as nickel silver, contains copper, zinc, and nickel. The nickel gives it a silver-like appearance and enhances its corrosion resistance. It is used in items like cutlery, jewelry, and musical instruments.
Specialized brass alloys like naval brass (40% zinc, 1% tin) offer enhanced corrosion resistance, making them ideal for marine applications. Cartridge brass (70% copper, 30% zinc) is used in ammunition and cold working applications. Free-cutting brass (with added lead) improves machinability and is widely used in hardware components, fittings, and valves.
To enhance the corrosion resistance of brass, several strategies can be employed. Firstly, optimizing the alloy composition is crucial. Increasing the copper content and reducing zinc can improve resistance to dezincification, a process where zinc is selectively leached out, leaving a porous structure. Adding elements like tin, manganese, or nickel can further enhance corrosion resistance, especially in aggressive environments such as saltwater.
Heat treatment processes, such as promoting an all-alpha phase microstructure through controlled heating and slow cooling, can make brass as resistant to dezincification as bronze alloys. Proper annealing also reduces internal stresses, enhancing both mechanical and corrosion properties.
Advanced surface protection techniques are highly effective. Applying nanocoatings or fillers like titanium dioxide creates a barrier that prevents corrosive elements from penetrating the brass surface. Electroplated coatings of zinc, nickel, or chrome provide long-term protection by acting sacrificially or as impermeable barriers. Polymeric and graphene oxide coatings offer robust protection against moisture, chemicals, and UV radiation, making them ideal for harsh environments.
Lastly, managing environmental factors such as exposure to chlorides, acids, or high humidity is essential for preventing corrosion and selecting appropriate protection methods. These combined approaches ensure the longevity and performance of brass components in various applications.
Brass, an alloy of copper and zinc, is widely utilized across various industries due to its desirable properties, such as corrosion resistance, durability, and aesthetic appeal. In plumbing, brass is commonly used for fittings, valves, taps, and pipe fittings because of its excellent corrosion resistance and malleability. In electrical applications, brass connectors and terminals benefit from high electrical conductivity and corrosion resistance.
Architecturally, brass is favored for lanterns, door hardware, roofing, and historical renovations, providing both durability and a classic appearance. Its antimicrobial properties make it ideal for bathroom fixtures and doorknobs in healthcare settings and public spaces, reducing bacterial spread. The automotive industry uses brass for components like radiators due to its lightweight and cost-effective nature. Additionally, brass is employed in tools and appliances for its low friction properties and in decorative items such as lamps, sculptures, and furniture for its attractive appearance and durability. These applications demonstrate brass’s versatility and importance in various functional and aesthetic roles.
To enhance brass properties step-by-step, follow these procedures:
By systematically applying these steps, brass properties such as hardness, strength, ductility, machinability, and corrosion resistance can be optimized for various industrial and decorative applications.

