Imagine the Eiffel Tower growing taller on a hot summer day and shrinking back during the winter months. This fascinating phenomenon is due to thermal expansion, a fundamental property of metals that has significant implications for engineering and material science. But what exactly is thermal expansion, and how do different metals respond to it? In this technical deep dive, we will unravel the complexities of thermal expansion in metals, exploring the underlying mechanisms, key factors influencing this behavior, and the critical role of the coefficient of thermal expansion (CTE). We will also delve into how thermal expansion impacts various engineering applications, from bridge construction to precision electronics, and examine how sustainable materials can mitigate its effects. Join us as we uncover the intricacies of thermal expansion and its pivotal role in modern engineering. Ready to expand your knowledge? Let’s dive in.
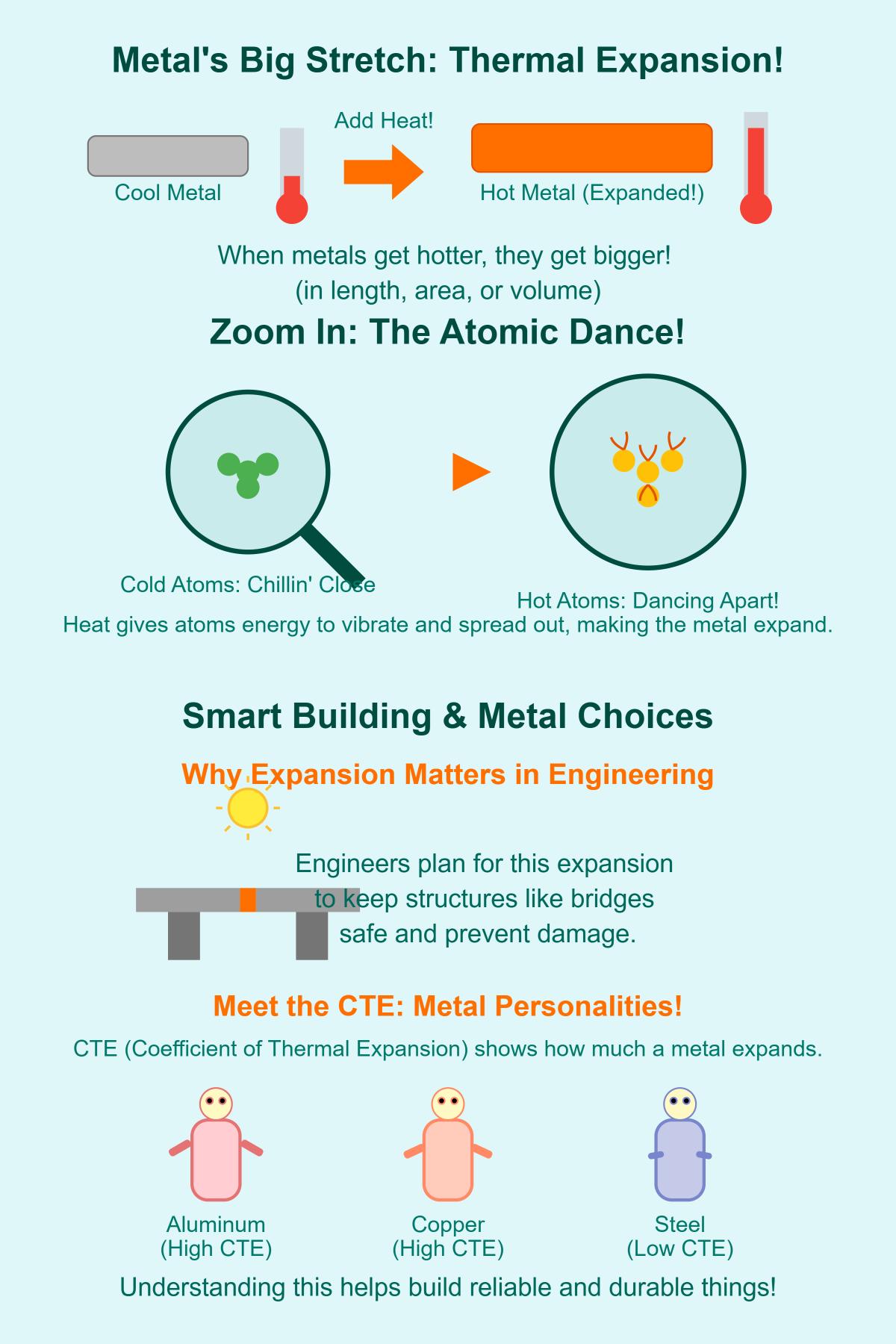
Thermal expansion is a basic property of materials, especially metals, which causes them to change in size when the temperature changes. At the atomic level, thermal expansion occurs because heating increases the kinetic energy of the atoms within a material. As the temperature rises, atoms vibrate more intensely and require more space, leading to an Thermal expansion can be classified into three main types: Linear Thermal Expansion, which involves the change in one dimension (length) of a material; Superficial Thermal Expansion, referring to the expansion in two dimensions, affecting the surface area; and Volume Expansion, encompassing expansion in all three dimensions, affecting the The Coefficient of Thermal Expansion (CTE) measures how much a material expands with each degree of temperature change. It is a critical parameter for engineers and designers, as it helps predict the dimensional changes that materials will undergo in response to temperature variations. The CTE is typically expressed in units of inverse temperature (e.g., 1/°C).
Understanding thermal expansion is essential for various engineering applications:
Thermal expansion in metals occurs because atoms within the material vibrate more vigorously when heated, causing them to move further apart and the material to expand. The extent of this expansion depends on the atomic structure and bonding within the metal.
In solid metals, thermal expansion is primarily driven by the anharmonicity of the interatomic potential. As the temperature rises, the potential energy curve of the atomic bonds becomes asymmetric. This causes the average distance between atoms to increase, leading to the expansion of the material. This phenomenon is well-understood for crystalline solids, where the regular arrangement of atoms allows for predictable expansion patterns.
Thermal expansion can be categorized based on the dimensions in which the expansion occurs: linear, superficial, and volumetric. Each type has distinct implications for the material’s behavior and its applications.
Linear thermal expansion refers to the change in length of a material when subjected to temperature changes. The linear thermal expansion coefficient (α) quantifies this change and is defined as the fractional change in length per degree of temperature change.
where:
Superficial or area thermal expansion involves changes in the surface area of a material. The superficial thermal expansion coefficient is approximately twice the linear coefficient for isotropic materials.
where:
Volumetric or volume thermal expansion encompasses changes in the entire volume of the material. The volumetric thermal expansion coefficient is approximately three times the linear coefficient for isotropic materials.
where:
Most metals expand when heated and contract when cooled, a behavior known as positive thermal expansion. However, some materials exhibit negative thermal expansion, contracting when heated due to unique interatomic interactions.
In liquid metals, atoms are not fixed in a lattice but move freely, leading to expansion primarily due to an increase in free volume rather than interatomic distances. This behavior results from the high atomic mobility and the nature of the liquid state.
Controlled expansion alloys are designed for specific thermal properties. For example, Invar has a very low expansion coefficient, making it ideal for applications requiring minimal dimensional changes. Other alloys are tailored to match the expansion of materials like glass or ceramics, used in electronic components, or have higher expansion coefficients for applications like thermostats.
Understanding the types and mechanisms of thermal expansion is essential for selecting the right materials and designing components that can withstand temperature variations without compromising performance or structural integrity.
Temperature changes are a key factor influencing the thermal expansion of metals. When a metal is heated, its atoms vibrate more vigorously, leading to an increase in the average distance between them. This results in the material expanding. Conversely, cooling a metal reduces atomic vibrations, causing the material to contract. The extent of thermal expansion or contraction is directly related to the temperature range the metal is subjected to.
Pure metals and alloys exhibit different thermal expansion characteristics due to variations in their atomic structures. Pure metals like aluminum and copper expand more than metals like steel, while alloys, which are combinations of metals with other elements, can have tailored expansion properties. The presence of different elements in an alloy can alter the thermal expansion characteristics by affecting the atomic structure and bonding forces. For example, Invar, an iron-nickel alloy, has a very low coefficient of thermal expansion (CTE), making it suitable for applications requiring minimal thermal expansion.
The crystal lattice structure of a metal, such as body-centered cubic (BCC), face-centered cubic (FCC), or hexagonal close-packed (HCP), plays a crucial role in its thermal expansion behavior.
Metals with a BCC structure, like iron at room temperature, generally exhibit moderate thermal expansion. The relatively open arrangement of atoms in BCC structures allows for more significant expansion when heated.
Metals with an FCC structure, such as aluminum and copper, typically have higher thermal expansion coefficients. The closely packed atoms in FCC structures result in greater atomic movement and expansion when subjected to temperature changes.
Metals with an HCP structure, like magnesium and titanium, often show anisotropic thermal expansion. This means their expansion can vary in different crystallographic directions due to the unique arrangement of atoms in HCP structures.
Impurities and defects in a metal can disrupt the regular atomic arrangement. This leads to localized changes in thermal expansion. Similarly, defects such as vacancies, dislocations, and grain boundaries can affect how a metal expands or contracts with temperature changes.
The bonding energy between atoms in a metal affects its thermal expansion. Metals with higher bonding energies generally have lower thermal expansion coefficients because their atoms are more tightly bound, resisting movement when heated. The potential energy well, which describes the energy landscape of atomic interactions, also influences thermal expansion. As the asymmetry of the potential energy well increases, so does the thermal expansion coefficient.
Mechanical constraints and external forces can impact metal expansion. If a metal is restricted in one or more dimensions, internal stresses may develop, affecting its performance and integrity. External forces like pressure can also influence atomic spacing and bonding, altering expansion behavior.
The Coefficient of Thermal Expansion (CTE) measures how much a material’s length changes with each degree of temperature change, usually expressed in μm/m·K (micrometers per meter per Kelvin) or ppm/°C (parts per million per degree Celsius). This value is crucial for engineers and materials scientists as it helps predict how materials will behave when subjected to temperature variations, which is vital for ensuring structural integrity and functionality in various applications.
Accurate CTE measurement is crucial for material selection and engineering design, and several techniques are used, each with unique benefits:
Dilatometry involves measuring the change in dimensions of a sample as it is heated or cooled. A dilatometer records the length change and calculates the CTE based on the temperature variation. This method is suitable for a wide range of materials and provides high precision over a broad temperature range.
Interferometry measures tiny length changes using light interference, providing high accuracy. This technique is particularly useful for materials that undergo very small dimensional changes. While highly precise, it is typically more complex and expensive than other methods.
TMA measures the deformation of a material under a controlled temperature program. For instance, TMA is widely used in the electronics industry to ensure components don’t warp under varying temperatures. ASTM Test Method E 831 outlines a standard procedure for using TMA to determine the linear thermal expansion of solid materials. This method is versatile and can be applied to a wide range of materials, including polymers, metals, and ceramics.
To facilitate the practical application of CTE in engineering designs, interactive tools and software are available. These tools allow users to input material properties and temperature ranges to calculate the expected dimensional changes due to thermal expansion. Such tools are valuable for engineers in predicting thermal stresses and ensuring the compatibility of different materials in composite structures.
Accurate CTE values are essential for several reasons:
Understanding and accurately measuring the CTE of materials is fundamental in fields ranging from construction and aerospace to electronics and precision engineering. It enables the development of reliable and high-performance products that can withstand the thermal stresses encountered in real-world applications.
Pure metals have unique thermal expansion behaviors due to their atomic structures and bonding characteristics. When heated, atoms in pure metals gain kinetic energy, causing them to vibrate more vigorously and move further apart, resulting in the metal expanding.
For example, aluminum has a relatively high coefficient of thermal expansion (CTE). This is due to its face-centered cubic (FCC) crystal structure, which allows significant atomic movement when the temperature rises. The open and symmetric nature of the FCC structure enables atoms to move more freely, leading to a greater increase in volume with temperature.
In contrast, metals like tungsten have a lower CTE. Tungsten’s body-centered cubic (BCC) structure has strong atomic bonds that restrict atomic movement, resulting in less expansion when heated. The relatively close-packed arrangement of atoms in the BCC structure and the strong bonds between them limit the atomic movement, causing less expansion.
Metal alloys are combinations of different metals and sometimes non-metals. The thermal expansion properties of alloys can be tailored based on their composition. By adding alloying elements, the atomic structure and bonding within the alloy are modified, which in turn affects its thermal expansion.
For example, Invar, an iron-nickel alloy, is known for its very low CTE, making it ideal for precision instruments and aerospace components where dimensional stability is crucial. The addition of nickel to iron changes the crystal structure and the interatomic forces in such a way that the expansion due to temperature increase is minimized.
Conversely, some alloys are designed to have higher CTE values for specific applications. For instance, certain alloys used in thermostats are engineered to expand significantly with temperature changes. This expansion is used to trigger mechanical movements within the thermostat, allowing it to regulate temperature.
A comparative study of different metals reveals significant differences in their thermal expansion characteristics. Metals can be classified into groups based on their CTE values.
High-expansion metals, such as aluminum and zinc, have CTE values typically in the range of 20-30 × 10⁻⁶/K. These metals are often used in applications where thermal expansion can be accommodated or even utilized, like in some types of heat exchangers.
Moderate-expansion metals, including copper and brass (an alloy of copper and zinc), have CTE values around 16-20 × 10⁻⁶/K. Copper’s good electrical conductivity and moderate thermal expansion make it suitable for electrical wiring and electronic components.
Low-expansion metals, such as steel and Invar, have CTE values lower than 10 × 10⁻⁶/K. Steel’s relatively low expansion and high strength make it a popular choice in construction, while Invar’s ultra-low expansion is ideal for precision engineering applications.
When designing structures or components that use multiple metals, it is essential to consider the differences in their thermal expansion characteristics. Mismatched CTE values can lead to thermal stresses, which may cause deformation, cracking, or failure of the structure over time.
In bridge construction, engineers must account for the thermal expansion of metal components to ensure the structure’s safety and functionality over a wide range of temperatures. Expansion joints are used to accommodate the dimensional changes that occur due to temperature variations. These joints allow the bridge to expand and contract without causing damage to the structure. For example, in large suspension bridges, the main cables and steel girders are subject to significant thermal expansion and contraction. Without proper expansion joints, the stresses generated by these dimensional changes could lead to structural failures such as cracking or buckling.
Small gaps are left between sections of railway tracks to accommodate thermal expansion and contraction. These gaps, known as expansion joints, prevent the tracks from warping or buckling in extreme temperatures by allowing the tracks to expand and contract. During hot weather, the tracks expand, and the gaps close slightly. In cold weather, the tracks contract, and the gaps widen. This design ensures that the tracks remain stable and safe for train travel. For instance, in regions with large temperature variations, such as deserts or high – altitude areas, the proper installation of expansion joints is crucial to prevent track damage and derailments.
Electrical power lines are designed with some slack to accommodate thermal expansion. When the temperature rises, the lines expand and sag. If the lines were too tight, they could break or pull down utility poles. By allowing the lines to have some slack, the effects of thermal expansion can be absorbed, ensuring continuous electricity supply. Additionally, the choice of materials for power lines is also influenced by their thermal expansion properties. Materials with relatively low coefficients of thermal expansion are preferred to minimize the amount of slack required and to maintain the stability of the lines.
Thermometers and thermostats use thermal expansion to measure and control temperature. In traditional mercury thermometers, the mercury expands when heated and moves up the tube, indicating an increase in temperature. The amount of expansion is proportional to the temperature change, allowing for accurate temperature measurement. Thermostats work on a similar principle. They use materials with known thermal expansion properties to control the flow of electricity or the operation of heating and cooling systems. When the temperature changes, the expansion or contraction of the material triggers a mechanical or electrical switch, which in turn adjusts the temperature of the environment.
Shrink fitting is a manufacturing process that joins two parts using thermal expansion. One part is heated until it expands, then fitted over another part. As it cools, it contracts, forming a tight fit. This method is commonly used in the assembly of machinery, such as engine components and bearings. For example, in the automotive industry, shrink fitting is used to install gears and pulleys onto shafts. The process ensures a strong and precise connection between the parts, improving the overall performance and longevity of the assembled components.
Recent research has focused on developing alloys with controlled thermal expansion properties. These tailored alloys can be designed to have zero thermal expansion or to match the expansion of other materials. For applications requiring high dimensional stability, such as in precision instruments and aerospace components, alloys with near – zero thermal expansion are ideal. In electronic devices, where different materials need to expand and contract in harmony to prevent damage, alloys can be engineered to match the thermal expansion of other components like glass or ceramics. This technology allows for the development of more reliable and high – performance products in various engineering fields.
Selecting materials based on their thermal expansion properties is crucial in ensuring the structural integrity and functionality of components exposed to varying temperatures. Several criteria guide this selection process:
The CTE measures how much a material expands or contracts with changes in temperature. Materials with low CTEs are preferred in applications requiring high dimensional stability, such as precision instruments and aerospace components. Conversely, materials with high CTEs can be advantageous in applications like thermostats, where expansion triggers mechanical actions.
The operating temperature range and mechanical properties such as strength, ductility, and toughness significantly influence material selection. Materials must be chosen based on their performance within the expected temperature fluctuations to avoid excessive expansion or contraction that could lead to mechanical failures.
When combining different materials, their CTEs must be compatible to prevent thermal stresses. This is particularly important in composite materials or assemblies where differential expansion could lead to delamination or component failure.
In bridge construction, materials like steel and concrete are commonly used. Steel, with its moderate CTE and high strength, is often chosen for structural components. Expansion joints are integrated to accommodate the thermal expansion of steel. These joints are designed to allow the bridge components to expand and contract with temperature changes without causing damage. They work by providing gaps or flexible connections that can absorb the movement, ensuring the bridge remains stable and functional.
In electronics, materials with matching CTEs are essential to maintain the integrity of connections and prevent thermal fatigue. For instance, in microelectronics, silicon chips are often bonded to substrates with similar thermal expansion properties to avoid cracking or delamination.
Aerospace components require materials with low CTEs to ensure dimensional stability under varying temperatures. Alloys like Invar are commonly used in precision instruments and structural components to minimize thermal expansion and maintain accuracy.
Compliance with ASTM standards ensures that materials meet the necessary thermal expansion requirements for specific applications. For instance, ASTM E228 outlines the standard test methods for determining the linear thermal expansion of solid materials, providing a benchmark for material selection.
Sustainable materials are increasingly important in modern engineering. Materials with low environmental impact and high recyclability are preferred. Selecting materials with suitable thermal expansion properties that also meet sustainability criteria contributes to the Materials with optimized thermal expansion properties can enhance energy efficiency. For example, in HVAC systems, materials that expand and contract predictably can improve the efficiency of thermal control mechanisms, reducing energy consumption.
Interactive tools and software are available to assist engineers in selecting materials based on thermal expansion properties. These tools allow users to input specific requirements and receive tailored recommendations. For example, an engineer could enter the desired CTE, temperature range, and mechanical properties, and the software would generate a list of suitable materials, providing a streamlined and efficient selection process.
Sustainable materials are essential in modern engineering because they reduce environmental impact and use resources more efficiently. These materials are designed to minimize their carbon footprint throughout their lifecycle, from production to disposal. By utilizing sustainable materials, industries can significantly lower greenhouse gas emissions and conserve natural resources, contributing to a healthier planet and a more sustainable future.
Choosing materials with energy-efficient properties is crucial for reducing operational energy consumption. Energy-efficient materials often have characteristics such as high thermal insulation, low thermal conductivity, and the ability to reflect or absorb specific wavelengths of radiation. These properties help maintain optimal thermal conditions, thereby reducing the need for additional heating or cooling.
Materials with high thermal insulation properties, such as certain ceramics and composite materials, are effective in maintaining temperature stability. They reduce the rate of heat transfer, ensuring that energy used for heating or cooling is not wasted. This is particularly important in the construction of buildings and industrial facilities, where maintaining a consistent internal temperature is vital for energy efficiency.
Alloys with low thermal conductivity, such as certain stainless steels and nickel-based alloys, help reduce energy loss in high-temperature environments. These materials are often used in applications where heat retention is crucial, such as in heat exchangers and industrial furnaces. By minimizing heat loss, these alloys contribute to more efficient energy use and lower operational costs.
The recyclability of materials is a key factor in their sustainability. Metals like aluminum and steel are highly recyclable, and their reuse significantly reduces the energy required for production. Recycling metals also decreases the need for mining raw materials, which has a substantial environmental impact.
Aluminum is one of the most recyclable materials. The recycling process consumes only about 5% of the energy required to produce primary aluminum from ore. This makes aluminum an excellent choice for sustainable engineering projects. Its high strength-to-weight ratio and corrosion resistance further enhance its suitability for various applications, from construction to automotive industries.
Steel is another highly recyclable material, with a recycling rate of over 85%. Moreover, recycled steel retains the same properties as new steel, making it a versatile and sustainable option for construction, infrastructure, and manufacturing. The use of recycled steel reduces energy consumption and greenhouse gas emissions, supporting sustainable development goals.
Recent advancements have led to the development of innovative alloys designed for sustainability and energy efficiency. These alloys are engineered to have specific thermal and mechanical properties that enhance their performance in various applications.
Invar, an iron-nickel alloy, is known for its minimal thermal expansion. This property makes it ideal for precision instruments and applications where dimensional stability is critical. The low thermal expansion reduces the energy needed for temperature control, contributing to energy efficiency.
Shape memory alloys, such as Nitinol (nickel-titanium), can return to their original shape upon heating. This unique property is used in various applications, including actuators and medical devices. The ability to undergo significant deformation and recover without energy-intensive processes makes these alloys highly energy-efficient.
Sustainable and energy-efficient materials play a crucial role in the development and optimization of renewable energy technologies. Materials with specific thermal and mechanical properties enhance the efficiency and durability of renewable energy systems.
The use of lightweight and durable materials, such as carbon fiber composites and high-strength steels, improves the efficiency and lifespan of wind turbines. These materials help reduce the overall weight of the turbine, which in turn decreases the stress on the structure and allows for larger and more efficient designs.
Materials with high thermal conductivity and durability, such as certain metals and ceramics, are used in the construction of solar panels. These materials enhance the thermal management of the panels, improving their energy conversion efficiency and extending their operational life.
The development of sustainable materials is an ongoing process, with continuous research focused on enhancing their properties and applications. Future trends include the creation of materials with even lower environmental impact, improved recyclability, and greater energy efficiency.
Nanomaterials, with their unique properties, offer significant potential for improving energy efficiency and sustainability. They can be engineered to have specific thermal and electrical properties, making them suitable for applications ranging from electronics to energy storage.
Bio-based materials, derived from renewable biological sources, are gaining attention for their sustainability. These materials can replace traditional petroleum-based products in various applications, reducing dependence on non-renewable resources and lowering environmental impact.
Below are answers to some frequently asked questions:
Thermal expansion in metals refers to the increase in the size of a metal when its temperature rises. This occurs because heating increases the kinetic energy of the metal’s atoms, causing them to vibrate more vigorously and move further apart. This phenomenon results in an expansion of the material’s length, area, or volume, depending on the dimensional constraints.
In practical terms, thermal expansion is critical in engineering and materials science, as it influences the design and performance of structures and devices. For instance, engineers must consider thermal expansion when designing bridges to prevent structural issues such as buckling or distortion. Different metals expand at different rates, characterized by their coefficient of thermal expansion (CTE). Metals like aluminum and copper have high CTEs, while metals like steel have lower CTEs.
Understanding thermal expansion is essential for ensuring the reliability and durability of engineering structures and components under varying temperature conditions.
Thermal expansion significantly impacts engineering structures by causing materials to expand when heated and contract when cooled. This phenomenon can lead to structural stress, potential distortion, and even cracking if not properly managed. The degree of expansion is determined by the material’s coefficient of thermal expansion (CTE), which varies among different metals and alloys.
Engineers must account for thermal expansion in their designs to maintain structural integrity. For example, expansion joints are used in bridges to accommodate the movement caused by temperature changes, preventing buckling or other forms of deformation. Selecting materials with appropriate thermal expansion properties and integrating them thoughtfully is crucial to mitigate thermal stresses.
Different metals respond to thermal expansion in distinct ways due to their unique coefficients of thermal expansion (CTE) and material properties. Metals like aluminum and copper have relatively high CTEs, meaning they expand more significantly with temperature increases. In contrast, steel has a lower CTE and expands less. Crystal structure, atomic bonding, and the presence of impurities or alloying elements also influence a metal’s response. For example, metals with more open crystal lattices expand more, and alloys can be engineered to have minimal thermal expansion, like Invar.
To calculate the coefficient of thermal expansion (CTE) for a material, you measure how its dimensions change as temperature varies. The CTE quantifies the fractional change in length per degree of temperature change and is crucial for predicting how materials behave in engineering applications. The linear thermal expansion coefficient (α) is calculated using the formula:
where ( ΔL ) is the change in length, ( L1 ) is the original length, and ( ΔT ) is the change in temperature.
For more comprehensive measurements, you might also consider the superficial (area) or volumetric expansion coefficients, which describe changes in area and volume respectively. Techniques such as dilatometry, interferometry, and thermomechanical analysis (TMA) are employed to measure these dimensional changes precisely. Understanding these coefficients helps ensure the reliability and performance of materials in thermal environments, making it a fundamental aspect of engineering design and materials science.
Several ASTM standards are relevant for measuring thermal expansion in engineering applications. These standards outline methods for determining the Coefficient of Linear Thermal Expansion (CLTE) and are essential for ensuring the stability and performance of structures subjected to temperature variations.
These ASTM standards help engineers select appropriate materials and design structures that can withstand thermal stress, ensuring structural integrity and functionality under varying temperature conditions.
Yes, there are sustainable materials with low thermal expansion, which are essential for applications requiring high dimensional stability and environmental friendliness. Recent advancements have led to the development of materials like cellulose nanofibers (CNFs), which exhibit low thermal expansion coefficients due to their strong interfiber hydrogen bonds and semi-crystalline structure. These materials are used in aerospace and sustainable construction for their high specific strength and thermal stability. Additionally, advanced ceramics such as aluminum nitride and zirconia, and super alloys like HRA929, offer low thermal expansion while maintaining high strength and stability at elevated temperatures, making them suitable for high-performance engineering applications. These materials provide both functional benefits and contribute to sustainability in engineering practices.

